Palliative efficacy and local control of conventional radiotherapy for lung metastases
Introduction
The lung is a common location for metastatic disease. Large autopsy series have shown pulmonary metastases in 20–54% of patients with extrathoracic malignancies (1). Depending on tumor size and location, respiratory symptoms may develop and include dyspnea, hemoptysis, cough, chest pain, SVC syndrome, and hoarseness. In addition to treating symptomatic disease, oncologists may recommend treatment for asymptomatic patients with the aim of local control (LC) of the lesion, in hopes of preventing future symptom development or deferring chemotherapy.
External beam radiotherapy is a commonly used treatment modality in the setting of metastatic cancer to the lung, as it can effectively relieve symptoms with a relatively rare incidence of serious side effects when using typical palliative doses. Typical palliative fractionation for lung metastases is similar to that used elsewhere in the body, with one common regimen being 30 Gy in 10 fractions. Compared to RT regimens for curative intent, such as 60 Gy in 30 fractions for non-small cell lung cancer (NSCLC), palliative radiotherapy schedules are intended to minimize the risk of serious toxicity and decrease overall length of treatment, while retaining sufficient tumoricidal effect to palliate symptoms in the short term.
The effectiveness of conventionally fractionated radiation therapy (CFRT) has been described for palliation of advanced NSCLC (2). However, no studies have specifically quantified the outcomes of palliative radiotherapy for secondary lung tumors, for which patient characteristics and radiation sensitivity may be different. In addition, increasing interest in “oligometastatic” patients raises the question of whether palliative-dose radiotherapy has a sufficiently durable effect, and can achieve meaningful LC, in patients with more likelihood of long-term survival. This question is particularly relevant with the advent of stereotactic body radiation therapy (SBRT), which has demonstrated excellent results in the treatment of early-stage NSCLC (3), and has demonstrated feasibility and effectiveness for lung metastases as well (4). SBRT is reasonably assumed to have superior LC than CFRT, but the magnitude of that advantage, and whether such advantage justifies increases in cost and potential toxicity, has not been investigated. Quantifying the outcomes of CFRT for lung metastases may aid clinicians in deciding when to recommend RT, and whether to consider SBRT in patients who may be technically eligible for it.
Therefore, we aimed to characterize the effectiveness of CFRT in terms of LC, the probability of initial palliation, and durability of palliative response. We also characterized the LC of a contemporary cohort of patients treated with SBRT for lung metastases, with the intention of making an exploratory comparison of LC between the two techniques.
Methods
Patient selection
This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center. Patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. Institutional databases were queried to identify patients receiving thoracic radiotherapy for secondary lung malignancies, treated at our institution between 1999 and 2014. SBRT for lung tumors was first implemented in 2004. We excluded patients younger than 18, patients receiving re-irradiation to a site of local failure (LF), and patients treated for disease limited to the mediastinum, ribs, or chest wall without involvement of lung parenchyma.
The CFRT cohort consisted of patients receiving a maximum of 50 Gy in conventional fractionation (maximum of 400 cGy per fraction), as doses greater than 50 Gy were associated with definitive rather than palliative intent. We only included CFRT patients who had sufficient follow-up to make a determination of either LC or symptom relief. LC was assessable if a CT or PET scan was performed at least one month after completion of RT. Palliation was assessable if there was documented clinician assessment of symptom severity within 3 months after RT. Twelve patients lacked follow-up of either kind and were excluded.
The CFRT group ultimately comprised 95 patients receiving 99 courses of treatment. The LC analysis comprised 79 courses of treatment (as 20 courses lacked follow-up for LC). The palliative analysis comprised 78 courses of treatment, as twenty courses were prescribed for asymptomatic disease and one additional course lacked available follow-up symptom assessment.
SBRT patients received a minimum of 500 cGy per fraction in five or fewer fractions, with CT image guidance at each fraction. Thirteen patients were excluded because they lacked available follow-up assessment of LC, leaving 88 patients receiving 91 courses of treatment. All analyses were performed on a per-course basis.
Treatment
CFRT patients were treated with various techniques depending on the clinical scenario and practitioner preference. 80 courses were delivered without customized immobilization devices, using simple opposed fields encompassing the symptomatic lesion with 1–2 cm margins. Six courses were delivered using 3D-conformal radiation therapy and 13 courses utilized intensity-modulated radiation therapy (IMRT). In either case patients were treated with arms raised, and a custom immobilization cradle. Typical margins for 3D-CRT were 1.2–1.5 cm from gross tumor volume (GTV) to planning tumor volume (PTV), or 2 cm to block edge. CFRT patients received a maximum total dose of 50 Gy and a maximum fractional dose of 400 cGy.
Patients treated with SBRT were treated with arms raised and a custom immobilization cradle. All received ≥500 cGy per fraction, with on-board CT guidance at each fraction. Four-dimensional CT simulation was employed to define the respiratory excursion of the GTV and generate an ITV, and the PTV consisted of an additional 7 gross tumor volume 8 mm expansion. Using coplanar beams (usually 4 to 7), IMRT was then prescribed to the 100% isodose line encompassing the PTV.
Data collection
All patient charts were retrospectively reviewed to obtain patient, disease, and treatment factors at the time of initiation of RT. Patient factors included age, gender, and performance status. Disease factors included site of origin, radioresistant histology (defined as melanoma, sarcomas, and renal cell carcinoma), and diameter of largest treated lung lesion. Treatment factors included total dose, number of fractions, and biologically equivalent dose (calculated using α/β ratio of 10).
We reviewed CFRT patient records to assess palliation, recording the indication for palliative RT in each case. Patients were scored as having achieved initial palliation if a clinician described partial or complete relief of at least one symptomatic indication within three months of completing RT. In patients who experienced palliation, we recorded the durability of palliation, defined as time from end of RT to the first record of symptom recurrence or worsening, if it occurred. We also characterized the probability of palliative response according to symptom type, in which case we analyzed the response of each symptom individually. LF was scored for both CFRT and SBRT patients and was defined as evidence of progression on CT or PET-CT imaging performed at least one month after the completion of RT, since we considered imaging within one month of RT too soon to assess radiographic response to therapy.
Statistical analysis
LF and durability of palliation were analyzed using competing-risks regression. The risk of each event was estimated using a cumulative incidence function that accounted for death without the event of interest. Cumulative incidence comparisons across subgroups were analyzed using Gray’s test. The Fine and Gray method was used for multivariate analyses. Kaplan-Meier analysis was used to estimate overall survival (OS), and patients who were still alive were censored at the date of last available follow-up. OS comparisons across subgroups were analyzed using the log-rank test. LF, durability of palliation, and OS were determined from the end of RT. Initial palliation (yes/no) was analyzed using logistic regression, and a two-sample proportion test was used to compare the rate of initial palliation according to symptomatic indication.
We performed a secondary analysis of LC in the combined CFRT and SBRT cohorts, considering this analysis exploratory in nature given the likelihood of significant selection bias and baseline differences between the groups. Patient and clinical characteristics were compared between the CFRT and SBRT cohorts using the chi-squared test and the Wilcoxon rank sum test. Candidate factors with P<0.10 on univariate analysis (UVA) were incorporated into a multivariate model (MVA). Statistical significance for all analyses was two-sided and used a 5% significance level (P<0.05). Statistical analyses were performed using R (version 3.1.0; R Development Core Team) with the “survival” and “cmprsk” packages.
Results
Patient characteristics
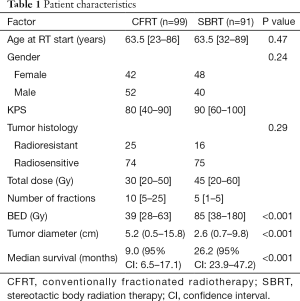
Median follow up of surviving patients at the time of analysis was 15.6 months (See Table 1). The median age at time of RT was 63 years (range, 23–89 years). The median CFRT dose was 30 Gy (range, 20–50 Gy) in a median 10 (range, 5–25) fractions. The median SBRT dose was 45 Gy (range, 20–60 Gy) in a median 5 (range, 1–5) fractions. The median tumor size for CFRT (5.2 cm, range, 0.5–15.8 cm) was significantly larger than for SBRT (2.6 cm, range, 0.7–9.8 cm, P<0.001). Median OS for CFRT and SBRT groups were 9.0 and 26.2 months, respectively (P<0.001).
Full table
Palliation
Fifty-eight courses of CFRT (74.4%) were associated with documented improvement of one or more symptoms after RT. Palliation rates for the most common indications were as follows: hemoptysis 86.2% (25/29), cough 70.6% (12/17), airway obstruction 65.8% (25/38), pain 63.6% (7/11) (Table 2). The rate of palliation for bleeding (26/30, 86.6%), which included hemoptysis and hemothorax, was higher than that for all other indications (44/66, 66.7%); this difference was borderline significant (P=0.072).

Full table
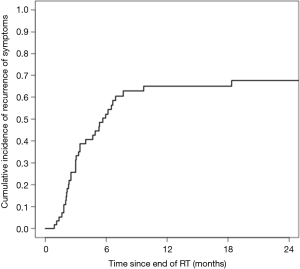
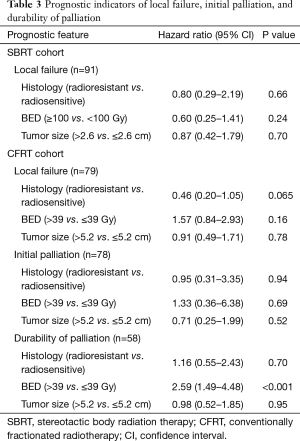
Among the 58 courses of CFRT leading to initial palliation, subsequent symptom recurrence was recorded in 36 cases (62.1%). The cumulative incidence of symptom recurrence was 52.4% at 6 months and 65.1% at 1 year (Figure 1). Patients receiving higher BED were less likely to have durable symptom relief (P<0.001). Otherwise, no prognostic factors were significantly associated with initial palliation or durability of palliation. Full analysis of prognostic indicators for palliative response are included in Table 3.
Full table
LC
In the CFRT cohort, cumulative incidence of LF at six months and one year was 32.1% and 43.2%, respectively. On UVA, tumor size >5.2 cm (median tumor size of the CFRT cohort), radioresistant histology, and BED >39 Gy (corresponding to 30 Gy in 10 fractions) were not significantly associated with LF.
In the SBRT cohort, cumulative incidence of LF at six months and one year was 5.8% and 19.5%, respectively. LF curves for each cohort are shown in Figure 2. Tumor size >2.6 cm (median size of SBRT cohort), radioresistant histology, and BED ≥100 Gy did not reach significance with respect to LF in the SBRT cohort. Analysis of prognostic indicators is included in Table 3.
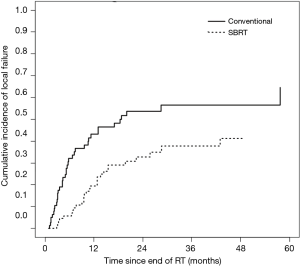
On exploratory analysis of LC in the combined cohort, tumor size >3.8 cm (median size of combined cohorts), BED ≤48 Gy (median BED of combined cohorts), and use of CFRT were significantly associated with LF. BED was excluded from multivariate modeling due to co-linearity with tumor size, and as tumor size was more significantly associated on UVA, we removed BED from our multivariate model. After multivariate modeling, only RT technique remained significant, with use of SBRT remaining an independent prognostic factor for decreased LF. Full analysis of the combined patient population is included in Table 4.
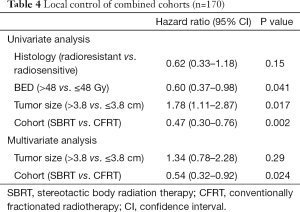
Full table
Discussion
To our knowledge, this report is the only published data quantifying the effectiveness of palliative-intent RT for secondary lung tumors. There is exists literature on the efficacy of conventionally fractionated palliative RT for patients with advanced NSCLC, but it remained unclear whether palliative RT for secondary lung metastases of other histologies has similar outcomes. Generally, studies in advanced NSCLC show that hemoptysis has the highest palliation rates, ranging from 76–97%, compared to a range of 50-84% for chest pain, 44–68% for airway obstruction, and 55–58% for cough (2,5,6). These are similar to our results of 86.2% for hemoptysis, 63.6% for chest pain, 65.8% for airway obstruction, and 70.6% for cough. Fewer studies report on the durability of palliative effect, even though this is also of obvious clinical import. One randomized study from the Medical Research Council showed a median duration of palliation of 50% or more of survival, with median survival of 6.2 months (5). Although we did not analyze the duration of palliation in the same way, this outcome appears consistent with our finding of 6-month incidence of symptom recurrence of 52.4%, in the context of median survival of 9.0 months. Our analysis did not reveal any factors clearly predictive of palliative outcomes, other than the observation that bleeding is particularly likely to respond to RT. The only exception was our finding that patients receiving higher-BED treatment were more likely to experience failure of palliation, a counterintuitive result. This may reflect selection bias (i.e., higher dose was prescribed because the practitioner suspected symptom relapse was more likely), or other factors that cannot be accounted for in retrospective research.
We also performed an exploratory comparison of LC between CFRT and SBRT, which is also the first such data to our knowledge. Given that fewer patients will be technically eligible for SBRT (which is only feasible for relatively small lesions), and that SBRT will typically be prescribed for patients with more favorable prognosis, we recognized that such a comparison would be inherently very limited. We using competing-risks analysis to adjust for the poorer prognosis and survival of the CFRT patients, and multivariate modeling to adjust for certain basic clinical factors, though these measures cannot fully account for the likely selection biases. Unsurprisingly, rates of LF were lower in the SBRT cohort than in the CFRT cohort. High rates of LC after SBRT for lung metastases have previously been demonstrated. Rusthoven et al. reported actuarial 1- and 2-year LC rates of 100% and 96%, respectively, in a prospective trial of SBRT for lung metastases (4). We observed a higher 1-year cumulative incidence of LF of 19.5%, which may be due to our lower median BED of 86 Gy10 (45 Gy in 5 fractions), compared to 180 Gy10 (60 Gy in 3 fractions) in the Rusthoven trial. In primary NSCLC, it is generally accepted that higher-BED regimens, particularly those ≥100 Gy10, are associated with improved LC, and it is reasonable to expect that higher SBRT doses would improve LC in metastatic lung tumors as well (7). Although SBRT patients receiving BED ≥100 Gy10 (n=29) had a lower incidence of LF in our cohort, this did not reach statistical significance (HR =0.60, P=0.24).
Of more interest to us was characterizing LC with CFRT, since LC generally is not analyzed or reported in studies of palliative-intent RT. Though it is unsurprising that longer-term LC rates with CFRT are modest, these estimates of CFRT control rates may provide clinicians with valuable guidance when treating better-prognosis patients for whom LC as well as palliation is desired. Our data suggests that patients expected to survive longer than six months have a greater than 50% chance of developing local progression after CFRT, which may prompt consideration of techniques more likely to achieve durable LC, such as SBRT.
The major limitations of this study are its retrospective nature and the heterogeneity of the patients included, with respect to disease characteristics and the clinical indication for treatment. We considered our comparison of CFRT and SBRT to be secondary and exploratory, given inherent baseline differences and selection biases. Nevertheless, the magnitude of differences in LC between the cohorts, and the relatively poor rates of LC with CFRT, do suggest that SBRT should be a consideration for patients with lower-volume lung metastases and relatively favorable prognosis.
We conclude that conventionally fractionated palliative lung RT offers a high probability of achieving initial symptom palliation due to lung metastases, corroborating the benefit that has been reported with advanced NSCLC. Patients with hemoptysis, in particular, are very likely to experience effective palliation with RT. However, the duration of palliative effect is limited in many cases, and most tumors will progress locally after CFRT within one year.
Acknowledgments
Funding: This research was supported by National Institutes of Health P30 CA008748.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (No. WA0269-14). Patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act.
References
- Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 2001;21:403-17. [Crossref] [PubMed]
- Erridge SC, Gaze MN, Price A, et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17:61-7. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70. [Crossref] [PubMed]
- Simpson JR, Francis ME, Perez-Tamayo R, et al. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys 1985;11:751-8. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]