Urinary cytokines/chemokines after magnetic resonance-guided high intensity focused ultrasound for palliative treatment of painful bone metastases
Introduction
Bone metastases are common among patients with advanced cancer and have been reported in up to 85% of cancer patients at autopsy (1). Pain is experienced by 50–75% of patients with bone metastases, representing a major source of morbidity amongst cancer patients (2).
External beam radiotherapy is the current standard treatment for patients with painful uncomplicated bone metastases (3). The overall pain response rate to external beam radiotherapy is approximately 60% (4). Radiotherapy re-treatment is limited by cumulative doses delivered to sensitive structures. Although cumulative effects do not limit other ablative techniques such as cryotherapy and percutaneous radiofrequency ablation, these techniques are invasive with risk of complications.
Magnetic resonance-guided high intensity focused ultrasound (MRgHIFU) is a new, non-invasive, outpatient treatment modality for painful bone metastases (5). It consists of a specially designed transducer that is used to focus a beam of ultrasound energy into a small volume at a specific target site in the body. The focused beam produces therapeutic hyperthermia in the target field (ablation is achieved when target tissue temperatures reach more than 57 °C), only harmlessly affecting the immediately surrounding tissue. Magnetic resonance (MR) imaging is used for two main purposes:
- Focus the ultrasound beam on the target field in the bone (the metastatic lesion and adjacent periosteum containing the nerves and vasculature for the tumor);
- Perform real-time thermal mapping at and around the target.
The mechanism of action of pain response is thought to be thermal periosteal denervation and/or thermal ablation of the tumor mass that diminishes pressure on the surrounding tissue (6-8). In addition, decrease in circulating immunosuppressive cytokines after MRgHIFU treatment is also thought to play a role in the overall reduction in pain response (9).
In this study, we aimed to analyze urinary cytokines/chemokines pattern after MRgHIFU for palliative treatment of painful bone metastases. The findings were compared to the cytokines/chemokines pattern post single 8 Gy fraction radiation from our previous study.
Methods
This was a single centre study that was conducted at Odette Cancer Centre (OCC), Sunnybrook Health Sciences Centre (SHSC)-Toronto, Canada. Ethics approval was obtained from the Research Ethics Board at SHSC. Patients with bone metastases who were planned to be treated with MRgHIFU were approached. Inclusion criteria included patient age ≥18 years, ability to give informed consent, patient weight <140 kg, radiologic evidence of bone metastases from any solid tumor, ability of patient to characterize pain specifically at the site of interest (target lesion) with pain score of ≥4 on a 0–10 point scale irrespective of medications, target lesion accessible for MRgHIFU procedure with maximum dimension ≤8 cm, target lesion as uncomplicated (i.e., no fracture/spinal cord compression/cauda equina syndrome/soft tissue component), target lesion visible by non-contrast MRI imaging, interface between bone and skin ≥1 cm from surface, ability to communicate sensation during MRgHIFU treatment, and MRgHIFU treatment date ≥2 weeks from most recent treatment of primary tumor or any chemotherapy.
We excluded patients with prior radiotherapy, surgery, ablative therapy, or other local therapy to target lesion, unable to characterize pain specifically at the site of interest, pregnant or nursing woman, target lesion as complicated (i.e., presence of one of fracture/spinal cord compression/cauda equina syndrome/soft tissue component). Target lesion <1 cm from nerve bundles/bladder/bowel, in contact with hollow viscera, and/or located in skull, spine (excluding sacrum which is allowed) or sternum were excluded. We also excluded the presence of scar along proposed MRgHIFU beam path, orthopaedic implant along proposed MRgHIFU beam path or at site of target lesion, serious cardiovascular, neurological, renal or hematological chronic disease, presence of active infection, inability of patient to tolerate required stationary position during treatment, and patients with allergy to MRI contrast agent or sedation.
Participating patients meeting the inclusion criteria were enrolled and informed consent was obtained. Urine samples were collected from patients with painful bone metastases 3 days before and 2 days after treatment with MRgHIFU. Patients received teaching on how to collect urine samples on their own. We decided to use the least invasive method to measure urinary cytokines/chemokines due to the underlying medical situation for our palliative patients. The Millipore Milliplex 42-Plex Cytokine/Chemokine Kit™ was used to measure urinary levels of a panel of cytokines/chemokines. Each urine sample was tested for pro-inflammatory cytokines and anti-inflammatory cytokines. In each urine sample we measured EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO, INFα2, INFγ, IL-1ra, IL-1α, IL-1β, IL-2, sIL-2Rα, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, IP-10, MCP-1, MCP-3, MDC, MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, sCD40L, TGFα, TNFα, TNFβ and VEGF as well as markers of bone turnover (N-telopeptides).
Statistical analysis
Descriptive analysis was conducted for pre-MRgHIFU, post-MRgHIFU, and MRgHIFU changes for each urinary cytokines using mean, standard deviation (SD), median, interquartile (Q1, Q3), and ranges in all patients. To compare pre-MRgHIFU and post-MRgHIFU cytokines levels in all patients and in patients with positive pain response, Wilcoxon signed rank test (non-parametric) was used. Two-sided P value <0.05 was considered statistically significant. For each individual patient, a list was performed for those urinary cytokines which significantly decreased post-MRgHIFU levels. Heat maps were conducted for 42 cytokine variables in all patients with pre-MRgHIFU and post-MRgHIFU. Box plots of significant urinary cytokines levels between pre-MRgHIFU and post-MRgHIFU were generated.
Similar descriptive analysis was also conducted for post-MRgHIFU (n=10) and post-radiation (n=28) for each “original” urinary, and Wilcoxon rank-sum test (non-parametric) to compare post-MRgHIFU and post-radiation cytokines levels. Kruskal-Wallis test (non-parametric) was used to compare patients from three groups: post-MRgHIFU, post-8 Gy fraction radiation with pain flare, and post-8 Gy fraction radiation with no pain flare for each urinary cytokine. All analyses were conducted using statistical analysis software (SAS version 9.4 for Windows).
Results
Ten patients were enrolled for the study from February 2011 to March 2012. They included four females and six males with a median age of 68.5 years with painful bone metastases from various primary tumors. Primary tumor sites included breast, prostate, pancreas, esophagus, orbit, lung, liver, and neuroendocrine. Table 1 summarizes the demographics of our patient population.
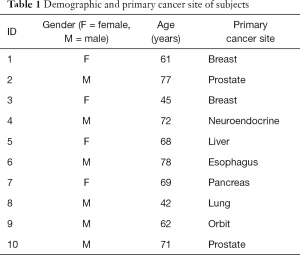
Full table
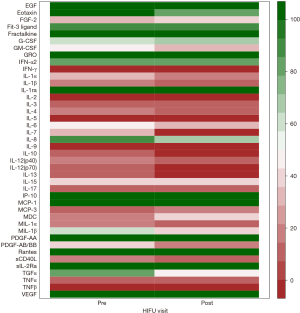
The following 15 cytokines were above the level of detection (LOD) in at least 50% of patients at both pre-MRgHIFU and post-MRgHIFU: EGF, eotaxin, Fit-3 ligand, fractalkine, G-CSF, GRO, IFNα2, IL-1ra, IL-8, IP-10, MCP-1, PDGF-AA, RANTES, sIL-2Rα, and VEGF. The heat map (Figure 1) demonstrates the changes in urinary cytokines pre-MRgHIFU and post-MRgHIFU. Nine urinary cytokines significantly decreased post-MRgHIFU, namely, eotaxin, GRO, IL-8, IL-13, IP-10, MCP-1, MIP-1β, RANTES, and sIL-2Rα (Table 2). Table 3 shows the nine urinary cytokines that significantly decreased post-MRgHIFU for each individual patient. Some patients showed a greater decrease in most cytokines post-MRgHIFU, e.g., patient ID =1, 3, 7, and 8. Figure 2 demonstrates boxplots for the selected nine cytokines; using original urinary cytokine levels at pre- and post-MRgHIFU treatment in all patients.
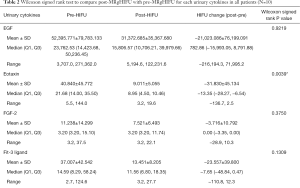
Full table
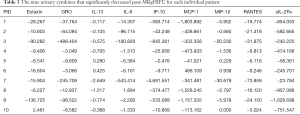
Full table
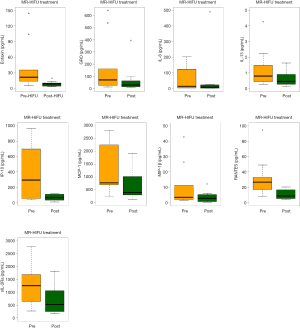
In our previous cytokines study with post-8 Gy fraction radiation treatment, we had 28 patients with days 1–5 post-8 Gy fraction radiation treatment for each urinary cytokines (10). When comparing the differences in urinary cytokines between post-MRgHIFU and post-8 Gy fraction radiation, there were significant difference between post-MRgHIFU and post-8 Gy fraction radiation on all urinary cytokines, except for FGF-2, IL-3, IL-6, IL-7, IL-8, IL-12(p40), IL-12(p70), IL-15, IL-17, MCP-1, MDC, MIP-1β, PDGF-AA, PDGF-AB/BB, sIL-2Rα, and TGFα. Patients with post-MRgHIFU are more likely to have higher cytokines on EGF, Eotaxin, Fit-3 ligand, fractalkine, G-CSF, GRO, IFNα2, IL-1β, IL-1ra, IP-10, M1P-1α, RANTES, and sCD40L, comparing to those with post-8 Gy fraction radiation. However, patients with post-MRgHIFU have significant lower cytokines on GM-CSF, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, MCP-3, TNFα, TNFβ, and VEGF, comparing to those with post-8 Gy fraction radiation.
None of our patients reported pain flare post-MRgHIFU treatment. We compared post-MRgHIFU from the current study with pain flare or no pain flare from our previous post-8 Gy fraction radiation study on each cytokine. Patients who reported pain flare post-radiation are more likely to have lower urinary cytokines levels for; EGF, Eotaxin, Fractalkine, GRO, IFNα2, IL-1α, IL-1β, IL-1ra, IP-10, RANTES, and sCD40L, compared to post-MRgHIFU patients. On the other hand, patients with no reported pain flare post-radiation are more likely to have significantly lower levels of Fit-3 Ligand cytokines compared to post-MRgHIFU. For other significant cytokines such as GM-CSF, IFN-γ, IL-4, IL-5, IL-9, IL-10, IL-13, MCP-3, TNFα, and TNFβ, post-radiation patients with pain flare are more likely to have higher cytokine values compared to post-MRgHIFU patients (Table 4).

Full table
In patients who had a positive pain response post-MRgHIFU, we correlated the patterns of cytokines post-MRgHIFU with pain response (5). We found no significant decrease in the cytokine patterns and pain response. We then compared cytokines level pre- and post-MRgHIFU in patients with positive pain response and found significant cytokine levels decreases in namely, GRO, IFN-γ, IP-10, IL-13, MCP-1, and RANTES (Table 5).
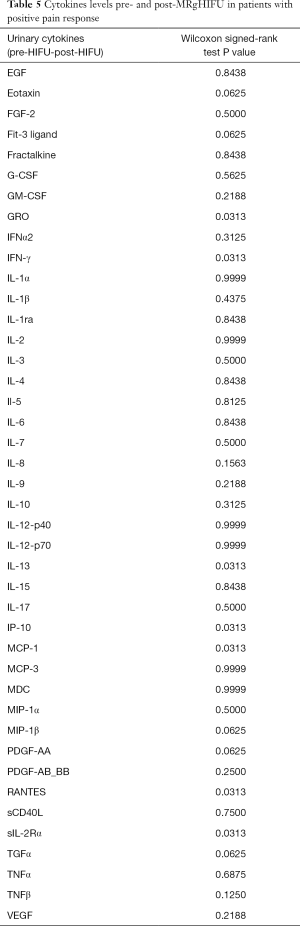
Full table
Discussion
The aim of this study was to analyze urinary cytokines/chemokines pattern after MRgHIFU for palliative treatment of painful bone metastases. The results showed that nine urinary cytokines significantly decreased post-MRgHIFU. They include; Eotaxin, GRO, IL-8, IL-13, IP-10, MCP-1, MIP-1β, RANTES, and sIL-2Rα. It is thought that MRgHIFU can decrease tumor-secreted immunosuppressive cytokine production; in addition it has a direct tumor destruction activity (9). These changes may reduce the effect of tumor-induced immunosuppression, and renew antitumor immunity after MRgHIFU in cancer patients.
Cytokines play a significant role in pain initiation and maintenance (10). Cytokines may be either pro- or anti-inflammatory; they are mainly produced by macrophages, neutrophils, and epithelial cells. Cytokines are mainly involved in the processes of angiogenesis, inflammation, wound healing, and tumorigenesis. They can down-regulate and inhibit the immune system of the host, contributing to the growth and progression of tumor (11-15).
MRgHIFU ablation causes direct destruction of tumor cells. This is thought to occur by activation of antitumor responses in the host following the ablation (16,17). As a result, this effect potentially allows the host immune system to control micro-metastases and decrease tumor recurrence at the local site following MRgHIFU treatment.
When comparing the urinary cytokines/chemokines pattern between post-MRgHIFU and post-8 Gy fraction radiation, we found significant differences between the cytokines pattern and no correlation could be made between the patterns seen in both treatment modalities (10).
Our study is not without any limitations; the most notable is our small sample size. Many of our enrolled patients were very sick with widespread metastatic disease. The clinical benefit of the decrease in cytokines post-MRgHIFU was not evaluated. The next step is to conduct a randomized clinical trial to assess the clinical significance of the changes in cytokines/chemokines pattern in patients with painful bone metastases.
In conclusion, our study showed that nine urinary cytokines significantly reduced post-MRgHIFU in patients with painful bone metastases. The significance of cytokines/chemokines pattern for palliative treatment of painful bone metastases is still unknown. Further research is required to confirm the possible correlation between decreased cytokines/chemokines pattern post-MRgHIFU with pain response in patients with painful bone metastases.
Acknowledgements
None.
Footnote
Conflicts of Interest: Charles Mougenot is employed by Philips Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval was obtained from the Research Ethics Board at Sunnybrook Health Sciences Centre (SHSC) (No. 260-2010) and written informed consent was obtained from all patients.
References
- Chow E, Hird A, Velikova G, et al. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer 2009;45:1146-52. [Crossref] [PubMed]
- Presutti R, Hird A, DeAngelis C, et al. Palliative radiotherapy of bone metastases and pain flare. J Pain Management 2011;4:105-15.
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Chan M, Dennis K, Huang Y, et al. Magnetic Resonance-Guided High-Intensity- Focused Ultrasound for Palliation of Painful Skeletal Metastases: A Pilot Study. Technol Cancer Res Treat 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience. Ann Oncol 2007;18:163-7. [Crossref] [PubMed]
- Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology 2008;249:355-63. [Crossref] [PubMed]
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 2009;16:140-6. [Crossref] [PubMed]
- Zhou Q, Zhu XQ, Zhang J, et al. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol 2008;34:81-7. [Crossref] [PubMed]
- Bushehri A, Chow E, Zhang L, et al. Urinary cytokines/chemokines pattern in patients with painful bone metastases undergoing external beam radiotherapy experiencing pain flare. Ann Palliat Med 2016;5:107-15. [Crossref] [PubMed]
- Chouaib S, Asselin-Paturel C, Mami-Chouaib F, et al. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 1997;18:493-7. [Crossref] [PubMed]
- Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004;64:2205-11. [Crossref] [PubMed]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol 2002;3:999-1005. [Crossref] [PubMed]
- Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 2006;16:3-15. [Crossref] [PubMed]
- Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 2004;92:13-27. [Crossref] [PubMed]
- Hu Z, Yang XY, Liu Y, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun 2005;335:124-31. [Crossref] [PubMed]
- Yang R, Reilly CR, Rescorla FJ, et al. Effects of high-intensity focused ultrasound in the treatment of experimental neuroblastoma. J Pediatr Surg 1992;27:246-50; discussion 250-1. [Crossref] [PubMed]


