Prophylaxis of radiation-induced nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials
Introduction
More than a century ago, Walsh (1) reported acute constitutional symptoms in an X-ray worker. In 1953, it was further characterized by Brown (2) with a distinct pattern of symptomatic disturbance after a single radiation dose, now known as radiation-induced nausea and vomiting (RINV). Of all patients receiving radiotherapy, 50–80% can develop RINV depending on the site of radiotherapy (3). In particular, those receiving total body irradiation (TBI), half body irradiation (HBI), and radiotherapy to upper abdomen are at a higher risk of RINV.
RINV is a distressing symptom and uncontrolled RINV can lead to potential complications such as dehydration and electrolyte disturbances. It could also result in interruption or even discontinuation of radiotherapy, thereby jeopardizing the treatment outcome. Recently, Poon et al. (4) showed that worse subjective experiences of RINV correlated with poorer quality of life (QoL). Therefore, awareness of RINV and more appropriate use of antiemetic agents could improve patients’ subjective experience, leading to better QoL.
The pathophysiology of RINV is uncertain and is currently postulated to be similar to that of chemotherapy induced nausea and vomiting (CINV) (5). The gastrointestinal tract is a major reservoir of serotonin and the serotonin pathway is thought to play a major role in RINV (4,6). Radiation induces damage to the gastrointestinal mucosa and causes release of serotonin (7), which activates 5-hydroxytryptamine-3 (5-HT3) receptors on afferent vagal nerves that transmit the signal to the brainstem vomiting centre, thus mediating nausea and vomiting (8). Therefore, 5-HT3 receptor antagonists (5-HT3 RA) are indicated in the treatment and prophylaxis of RINV.
Current antiemetic guidelines classify radiotherapy treatment into minimal, low, moderate, and high risk of RINV, mainly based on the anatomical site being irradiated (3,9). Apart from the site of radiation, the incidence and severity of RINV is affected by other treatment factors (dose per fraction, total dose, radiation field size, radiation technique and concurrent chemotherapy) (3,10) and patient factors (previous CINV, gender, age, anxiety and daily alcohol consumption) (11).
For patients at high emetogenic risk (i.e., receiving TBI or total nodal irradiation), current guidelines recommend prophylaxis with a 5HT3 RA and a short course of dexamethasone (3,9). For patients at moderate emetogenic risk (i.e., receiving radiotherapy to the upper abdomen or HBI), the guidelines recommend prophylaxis with a 5HT3 RA plus an optional short course of dexamethasone. For patients at low emetogenic risk, the guidelines recommend prophylaxis or rescue with a 5-HT3 RA. For patients at minimal emetogenic risk, the recommendation is rescue with a dopamine receptor antagonist or 5HT3 RA.
Despite the publication of various guidelines on the management of RINV, the use of antiemetics is often reported to be suboptimal. Maranzano et al. (10) prospectively analyzed 1,020 patients undergoing radiotherapy in 45 Italian radiation oncology centres. An antiemetic was only prescribed to a minority (17%) of patients, despite the fact that 27.9% of patients had nausea and/or vomiting. Enblom et al. (12) reported that one third of patients with radiation induced nausea considered their antiemetic treatment insufficient. More recently, a survey on international pattern of practices by Dennis et al. (11) noted the low awareness of antiemetic guidelines among 1,022 radiation oncologists from 12 countries and the insufficient recommendation of antiemetics compared with guideline recommendations, especially for moderate risk cases.
The objective of our systematic review was to evaluate the efficacy of various antiemetics in the prophylaxis of RINV among randomized controlled trials (RCTs).
Methods
Search strategy
A literature search was performed on Ovid MEDLINE (1946 to September 2015), EMBASE (1947 to September 2015), and Cochrane CENTRAL (until September 2015) databases. The following keywords were used: “neoplasms”, “neoplasm”, “cancer”, “tumor”, “tumour”, “radiotherapy”, “nausea”, “vomiting” and “drug therapy”. Reference lists of identified articles were also searched to find additional studies.
Study selection
We included all RCTs that evaluated the efficacy of prophylaxis for RINV in patients receiving radiotherapy to the abdomen and/or pelvic region, including TBI. We only included articles that were written in English and studies that were published. We excluded studies where concomitant chemotherapy was used to avoid the confounding effect of chemotherapy on nausea and vomiting. We also excluded studies with previously published duplicate data.
Four reviewers (Wing S. Li, Joanne M. van der Velden, Vithusha Ganesh and Sherlyn Vuong) were organized in pairs to screen the titles and abstracts of identified citations independently. Full texts of citations were obtained if judged as potentially eligible by at least one reviewer. Full texts were then screened by the reviewers and selected according to eligibility criteria. Disagreements were resolved by consensus.
Endpoints
The primary endpoints were complete control of nausea (defined as no nausea episodes) and complete control of vomiting (defined as no emetic episodes) during the acute phase (from the first day to last day of radiotherapy) and delayed phase (study period after completion of radiotherapy). Secondary endpoints included the use of rescue medication, QoL and incidence of adverse events.
Data analysis
Statistical analysis was performed using Review Manager (RevMan 5.3) from Cochrane IMS. The Mantel-Haenszel method was applied and a random effects analysis model was used to generate odds ratios (OR), absolute risk differences (RD), and accompanying 95% confidence intervals (CI). A P value of less than 0.05 was considered statistically significant in the test for overall effect, whereas heterogeneity test with a P value greater than 0.05 was considered suitable.
Results
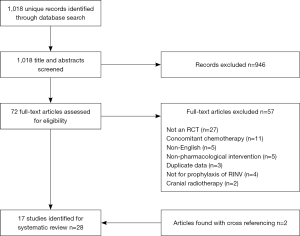
The search yielded 1,018 unique citations. After title and abstract screening, we retrieved and screened the full-texts of 72 articles. Two references were identified for the study by Collis et al. (13,14). Reference (14) was the interim analysis of the study with description of the methodology while reference (13) reported the final analysis of the study. Two additional studies were retrieved by cross-referencing. These two studies were not identified in our primary search because the keyword ‘cancer’ or ‘neoplasm’ was not selected by the authors of the studies (15,16). In total, 17 RCTs were included in this systematic review (Figure 1).
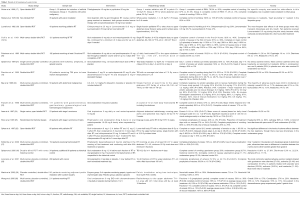
The characteristics of the included 17 RCTs are summarized in Table 1. Fourteen studies included patients receiving radiotherapy to the abdomen and/or pelvic regions. Among these 14 RCTs, three studies (22,23,28) compared a 5HT3 RA against placebo, three studies (13,20,24) compared a 5HT3 RA against a dopamine receptor antagonist and one study (25) compared a 5HT3 RA against rescue therapy. One study (30) compared the combination of a 5HT3 RA and dexamethasone against a 5HT3 RA plus placebo, another study (26) compared a 5HT3 RA against chlorpromazine plus dexamethasone, and one other study (27) compared dexamethasone against placebo. The remaining four studies (17-19,30) investigated other agents including thiethylperazine (an antiemetic of phenothiazine group acting as a dopamine receptor antagonist), pyridoxine, ibuprofen [non-steroidal anti-inflammatory drug (NSAID)], levonatradol (cannabinoid) and enzyme capsules (containing papain, trypsin and chymotrypsin). Beyond abdominal and/or pelvic radiation, the other three RCTs (15,16,21) included patients receiving TBI. Among these three, one compared a 5HT3 RA against placebo (21), one compared a 5HT3 RA with the combination of metoclopramide, dexamethasone and lorazepam (15), and the remaining study (16) compared two 5HT3 RAs, ondansetron versus granisetron, against each other.
Full table
Complete control of RINV in the acute phase
Radiotherapy to the abdomen and/or pelvic regions
A meta-analysis was not performed for all studies that compared a 5HT3 RA against another antiemetic in view of the high degree of clinical heterogeneity among all studies that compared a 5HT3 RA with different therapy groups. To facilitate the comparison of 5HT3 RAs with different antiemetics, the analysis was separated by comparison groups.
5HT3 RA versus placebo
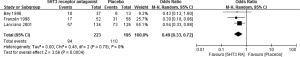
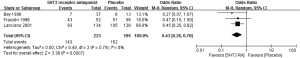
Meta-analysis of three RCTs (22,23,28) showed a significant benefit of 5HT3 RA over placebo in both complete control of vomiting (OR 0.49; 95% CI: 0.33–0.72, Figure 2) and complete control of nausea (OR 0.43; 95% CI: 0.26–0.70, Figure 3).
5HT3RA versus dopamine receptor antagonist
Meta-analysis of three RCTs (13,20,24) demonstrated a significant benefit of 5HT3 RA over dopamine receptor antagonists (metoclopramide, prochlorperazine) in complete control of vomiting (OR 0.17; 95% CI: 0.05–0.58, Figure 4). 5HT3 RA was also superior to dopamine receptor antagonists in complete control of nausea in two RCTs (13,20) (OR 0.46; 95% CI: 0.24–0.88, Figure 5). One study (24) was excluded from the meta-analysis of complete control of nausea because it did not report the proportion of patients that had no nausea during the study period. Instead, it reported a higher mean score of nausea [based on daily visual analogue scale (VAS)] and more patients suffered from significant nausea (defined by a score of >25 mm on the VAS) in the metoclopramide group compared with the tropisetron group.


5HT3 RA versus rescue therapy
Only one study was identified. In patients receiving multiple-fraction dog-leg or para-aortic radiotherapy, Khoo et al. (25) showed that prophylactic oral ondansetron was better than rescue therapy with metoclopramide in complete control of nausea (67% vs. 34%, P=0.02). There was a trend towards better complete control of vomiting (80% vs. 30%, P=0.06)
5HT3 RA versus dopamine receptor antagonist plus dexamethasone
The single study by Sykes et al. (26) demonstrated that oral ondansetron was more effective in complete or major control (0–2 emetic episodes) of vomiting (93.9% vs. 34.4%, P< 0.001) and complete control of nausea (70% vs. 28% P< 0.001) than the combination therapy of chlorpromazine and dexamethasone in patients undergoing single fraction radiotherapy to upper abdomen or lower HBI.
5HT3 RA plus dexamethasone versus 5HT3 RA plus placebo
Wong et al. (30) investigated the addition of a short course of dexamethasone (fractions 1–5) to ondansetron in patients receiving radiotherapy (≥15 fractions) to the upper abdomen. During the prophylactic period (fractions 1–5), the dexamethasone arm showed a trend of better complete control of nausea (50% vs. 38%, P=0.06), while complete control of vomiting was similar (78% vs. 71%, P=0.14). During the overall study period (fractions 1–15), complete control of vomiting was better (23% vs. 12%, P=0.02) and the average nausea score (using a 4-point scale) was lower (0.28 vs. 0.39, P=0.03) in the dexamethasone arm, while complete control of nausea was similar (15% vs. 9%, P=0.14).
Dexamethasone versus placebo
Kirkbride et al. (27) reported better complete control of vomiting (70% vs. 49%, P=0.025) in patients receiving dexamethasone prophylaxis during multiple-fraction radiotherapy to the upper abdomen.
Other agents
Sicher et al. (17) investigated the efficacy of thiethylperazine against pyridoxine in patients undergoing ovarian ablation (group 1) or radiotherapy to whole abdomen and pelvis (group 2). It was shown that thiethylperazine was more effective in the complete control of RINV (78.3% vs. 50%, P< 0.01 in group 1 and 71.4% vs. 0%, P< 0.01 in group 2) than pyridoxine. In patients receiving whole pelvic irradiation, Stryker et al. (18) reported that patients on oral ibuprofen had better complete control of vomiting (100% vs. 73%, P< 0.05) but similar complete control of nausea (65% vs. 60%) compared to the control group with no prophylactic treatment. In the study by Lucraft et al. (19), levonantradol had no advantage over chlorpromazine in the complete control of vomiting [41.4% vs. 50%, P value not reported (NR)] in patients receiving single fraction palliative radiotherapy to the upper abdomen. Finally, Martin et al. (29) compared enzyme capsules (containing papain, trypsin and chymotrypsin) with placebo in patients receiving pelvic irradiation. There was no difference in the control of vomiting (none/mild vomiting) (100% vs. 97%, P value NR) or the control of nausea (none/mild nausea) (93% vs. 93%) between the two groups.
TBI
A pooled analysis was not performed because the three RCTs compared 5HT RAs with different agents.
5HT3 RA versus placebo
Spitzer et al. (21) showed that patients in the ondansetron arm had better control of vomiting (≤2 episodes) (50% vs. 0%, P=0.012) during four days of TBI.
5HT3 RA versus the combination of metoclopramide, dexamethasone and lorazepam
Prentice et al. (15) demonstrated better complete control of vomiting (53% vs. 13.3%, P=0.001) during the acute phase in the granisetron arm relative to combination treatment.
Granisetron versus ondansetron
Complete control of vomiting (33% vs. 26.7%) and complete control of nausea (11.1% vs. 13.3%) were similar between the two groups as reported in the study by Spitzer et al. (16).
Complete control of RINV in the delayed phase
Only three studies evaluated control of delayed nausea and vomiting. Collis et al. (13) reported that ondansetron resulted in better complete or major control (≤2 episodes) of vomiting (day 2 98% vs. 86%; day 3 100% vs. 93%; day 4 98% vs. 91%; day 5 98% vs. 96%) yet similar control of (none or mild) nausea (day 2 82% vs. 76%; day 3 75% vs. 75%; day 4 79% vs. 84%; day 5 74% vs. 83%) when compared with metoclopramide in patients receiving single fraction radiotherapy to the upper abdomen. Sykes et al. (26) showed better complete or major control (0–2 episodes) of vomiting on days 2–4 (day 2: 96.2% vs. 42.9%; day 3 96.2% vs. 39.3%; day 4 96% vs. 37%, P< 0.001) with ondansetron relative to chlorpromazine plus dexamethasone in patients receiving single fraction HBI or radiotherapy to the upper lumbar spine. Finally, Prentice et al. (15) showed that granisetron was only slightly better than the combination therapy of metoclopramide, dexamethasone and lorazepam in complete control of vomiting over 7 days (13.3% vs. 6.7%, P=0.004) after TBI on day 1.
Use of rescue medication
Five RCTs investigated the use of rescue medication (15,16,24,27,30). Aass et al. (24) reported a similar proportion of patients (18.2% vs. 25%, P value NR) requiring rescue medication in both tropisetron and metoclopramide groups. Wong et al. (30) demonstrated a trend towards less use of rescue medications (70% vs. 80%, P=0.09) with addition of dexamethasone to ondansetron. In the study by Kirkbride et al. (27), it appeared that less patients in the dexamethasone group required rescue medication than those in the placebo group (29% vs. 43%, P=0.125). In patients receiving TBI, Spitzer et al. (16) reported that fewer patients in the ondansetron group required additional rescue medication than those in the placebo group (40% vs. 0%, P value NR). Prentice et al. (15) also showed that significantly fewer patients receiving TBI in the granisetron group required additional rescue medication compared with the combination therapy of metoclopramide, dexamethasone and lorazepam (46.7% vs. 93.3%, P=0.05).
QoL
Four trials evaluated QoL in patients receiving antiemetics during the study period. Franzén et al. (23) used the EORTC QLQ-C30 questionnaire for evaluation before the start of treatment, at two weeks and at the end of treatment. Patients in the ondansetron group reported better functioning, global QoL and lower symptom levels at week 2 than those in the placebo group. Wong et al. (30) evaluated QoL using the EORTC QLQ-C30 questionnaire as well. With the addition of dexamethasone to ondansetron, there were significant benefits in appetite, nausea and vomiting and a trend favouring global QoL improvement. However, there were marginally worse outcomes in the sleep and constipation scales. Sykes et al. (26) used the Functional Living Index Cancer (FLIC) and Functional Living Index Emesis (ELIE) QoL questionnaires before treatment and at the end of study. There was no difference for the FLIC questionnaire, but there was a difference in favour of the ondansetron group over the chlorpromazine plus dexamethasone group for the FLIE questionnaire. Kirkbride et al. (27) also utilized the EORTC QLQ-C30 questionnaire for evaluating QoL outcomes. There was no difference in global QoL between the two arms, but patients in the dexamethasone group had better scores in the domains of nausea/vomiting and appetite but a lower score in the domain of sleep when compared with patients in the placebo group.
Adverse events
The adverse events of various agents are summarized in Table 1. A pooled analysis could not be performed among the clinical studies as the results were reported in different ways. Two studies reported a higher overall rate of adverse events in the 5HT3 RA group over the placebo group [43.2% vs. 7.6%, P value NR in the study by Bey et al. (22) and 82.1% vs. 69.2%, P value NR in the study by Lanciano et al. (28)]. In general, the adverse events were usually mild to moderate in severity. There was no grade 5 or serious toxicity reported. The more commonly reported side-effects of 5HT3 RAs include headache (2.7–53.3%) and constipation (11.2–20%). Other less commonly reported adverse events are abdominal pain (8.1%), asthenia (11.1–25.4%), drowsiness (33.3%), and tachycardia (5.4%). The side effect of constipation with 5HT RAs could be advantageous in patients receiving radiotherapy to the abdomen /pelvic region, where diarrhea can be a side effect from radiation. Khoo et al. (25) reported a trend of reduced diarrhea with ondansetron in patients receiving para-aortic field radiotherapy.
Discussion
Our systematic review demonstrated that 5HT3 RA was significantly more efficacious than all other therapy groups (placebo, dopamine receptor antagonist, rescue therapy and dopamine receptor antagonist plus dexamethasone) in the prevention of acute RINV among patients receiving single or multiple fraction radiotherapy to the abdomen/pelvis. Limited data showed that 5HT3 RA was also better than dopamine receptor antagonist or dopamine receptor antagonist plus dexamethasone in the prophylaxis of radiation induced vomiting during the delayed phase among patients receiving single fraction radiotherapy to the upper abdomen.
The addition of dexamethasone to 5HT3 RA gives a slight benefit in the prophylaxis of RINV when compared with 5HT3 RA plus placebo. One study (30) showed better complete control of nausea during the prophylactic period with dexamethasone and better complete control of vomiting during the overall study period in patients receiving multiple-fraction radiotherapy to the upper abdomen.
Among patients receiving TBI, 5HT3 RA was also more efficacious than other agents (placebo, combination of metoclopramide, dexamethasone and lorazepam) in the prevention of RT induced vomiting during the acute phase (15,21). During the delayed phase, one study (15) showed that 5HT3 RA was better than the combination of metoclopramide, dexamethasone and lorazepam in the complete control of vomiting.
There appears to be a higher incidence of overall adverse events in patients receiving 5HT3 RA than the placebo arm, as reported in two studies (22,28). However, the reported side-effects of 5HT3 RA are mostly mild to moderate in severity only.
Among the four studies that investigated QoL, two reported better QoL in the 5HT3 RA arm over placebo or dopamine receptor antagonist plus dexamethasone (23,26). QoL was also more favourable in patients receiving 5HT3 RA plus dexamethasone compared with 5HT3 RA plus placebo (30) and dexamethasone compared with placebo (27).
Our systematic review supports the recommendation of current guidelines (3,9). 5HT3 RA is the antiemetic of choice in the prophylaxis of RINV in patients receiving radiotherapy at high and moderate emetogenic risk. The addition of a short course of dexamethasone to a 5-HT3 RA provides extra benefit in patients receiving radiotherapy to the upper abdomen. Based upon evidence from the moderate emetogenic risk group, patients at high emetogenic risk should also receive a 5-HT3 RA plus dexamethasone as prophylaxis.
Moreover, there is concrete evidence that prophylaxis of RINV was more effective than placebo (21-23,27,28) or rescue therapy (25) among patients receiving radiotherapy to the upper abdomen or TBI. Therefore, radiation oncologists are encouraged to be aware of current guidelines on RINV and follow the recommendations in daily practice, in order to maximize the control of RINV and preserve the QoL of patients.
Despite the use of 5HT3 RA in the prophylaxis of RINV, the complete control of nausea and vomiting is still suboptimal, especially in patients receiving multiple-fraction radiotherapy to the upper abdomen (complete control of nausea 9–30.6%; complete control of vomiting 12–67%) (20,23,28) and TBI (complete control of acute vomiting 26.7–53%; complete control of delayed vomiting 13.3%) (15,16).
Recent studies have shown that aprepitant, a substance P neurokinin 1 receptor antagonist, has a promising effect in the prophylaxis of RINV when combined with a 5HT3 RA. Dennis et al. (31) showed that the combination of aprepitant and granisetron was efficacious and safe for the prophylaxis of both acute and delayed RINV in patients receiving moderately emetogenic radiotherapy for thoracolumbar bone metastases (100% complete control of RINV in single fraction and 67% complete control of RINV in multiple fractions during acute phase). In a recent RCT, Emami et al. (32) reported that the combination of ondansetron and aprepitant was significantly better than ondansetron alone in the prevention of RINV among patients receiving radiotherapy to the abdomen (OR =0.13; P< 0.05). This trial was not included in our systematic review as some of the patients received concurrent chemotherapy with radiation. Therefore, future RCTs should investigate the benefit of adding aprepitant to 5HT3 RAs in the prophylaxis of RINV.
More recently, Wong et al. (33) reported ondansetron rapidly dissolving film was effective for the prophylaxis of RINV. This new formulation of ondansetron is bioequivalent to oral ondansetron formulations. It may be particularly useful for secondary prophylaxis in patients who have pre-existing nausea or vomiting, when swallowing of oral pills could be difficult. The study showed that the rates of overall control of nausea and vomiting for primary prophylaxis were 88% and 93% during the acute phase and 73% and 75% during the delayed phase, respectively. The rates of overall control of nausea and vomiting for secondary prophylaxis were both 100% during the acute phase and 50% during the delayed phase. Future trials could also investigate whether ondansetron rapidly dissolving film is more effective than the oral formulation in the prevention of RINV.
Our systematic review has some limitations. There is a potential publication bias since we included results from published papers only. Also, inclusion of articles written in English only could lead to selection bias. There is heterogeneity among the RCTs including variations in the definition of study endpoints, radiation treatment details (dose fractionation, total dose and radiation volume) and patient population (various types of cancer and extent of involvement). This heterogeneity limited our ability to perform a meta-analysis on certain endpoints. Further, some of the studies had small sample sizes (n=15–30) while other studies dated back to the 1970s and 1980s. There is also a chance of ecological fallacy since individual patient data was not available in performing the meta-analysis.
Conclusions
5HT3 RA is superior to placebo and other agents in the prevention of RINV among patients receiving single fraction or multiple-fraction radiotherapy to the abdomen and pelvis. The addition of dexamethasone to 5HT3 RA gives a modest improvement in the prophylaxis of RINV. During TBI, 5HT3 RA is also more efficacious than other agents such as dopamine receptor antagonists alone, dopamine receptor antagonists plus dexamethasone, lorazepam in the prevention of radiation-induced vomiting. However, there is still room for improvement in the complete control of nausea and vomiting, especially in patients receiving multiple-fraction radiotherapy to the upper abdomen and TBI. Future RCTs should investigate the efficacy and safety of substance P neurokinin 1 receptor antagonist in addition to 5HT3 RA for the prophylaxis of RINV during both acute and delayed phases.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Walsh D. Deep Tissue Traumatism from Roentgen Ray Exposure. Br Med J 1897;2:272-3. [Crossref] [PubMed]
- Brown WM. Symptomatic disturbance after single therapeutic dose of x rays; its relationship to the general radiation syndrome. Br Med J 1953;1:802-5. [Crossref] [PubMed]
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21 Suppl 5:v232-43. [Crossref] [PubMed]
- Poon M, Hwang J, Dennis K, et al. A novel prospective descriptive analysis of nausea and vomiting among patients receiving gastrointestinal radiation therapy. Support Care Cancer 2016;24:1545-61. [Crossref] [PubMed]
- Feyer P, Jahn F, Jordan K. Prophylactic Management of Radiation-Induced Nausea and Vomiting. Biomed Res Int 2015;2015:893013. [Crossref] [PubMed]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Ann Rev Med 2009;60:355-66. [Crossref] [PubMed]
- Scarantino CW, Ornitz RD, Hoffman LG, et al. On the mechanism of radiation-induced emesis: the role of serotonin. Int J Radiat Oncol Biol Phys 1994;30:825-30. [Crossref] [PubMed]
- Minami M, Endo T, Hirafuji M, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 2003;99:149-65. [Crossref] [PubMed]
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189-98. [Crossref] [PubMed]
- Maranzano E, De Angelis V, Pergolizzi S, et al. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol 2010;94:36-41. [Crossref] [PubMed]
- Dennis K, Zhang L, Lutz S, et al. International patterns of practice in the management of radiation therapy-induced nausea and vomiting. Int J Radiat Oncol Biol Phys 2012;84:e49-60. [Crossref] [PubMed]
- Enblom A, Bergius Axelsson B, et al. One third of patients with radiotherapy-induced nausea consider their antiemetic treatment insufficient. Support Care Cancer 2009;17:23-32. [Crossref] [PubMed]
- The final assessment of a randomized double-blind comparative study of ondansetron vs. metoclopramide in the prevention of nausea and vomiting following high-dose upper abdominal irradiation. Clin Oncol (R Coll Radiol) 1991;3:241-2. [Crossref] [PubMed]
- Priestman TJ, Roberts JT, Lucraft H, et al. Results of a randomized, double-blind comparative study of ondansetronand metoclopramide in the prevention of nausea and vomiting following high-dose upper abdominal irradiation. Clin Oncol (R Coll Radiol) 1990;2:71-5. [Crossref] [PubMed]
- Prentice HG, Cunningham S, Gandhi L, et al. Granisetron in the prevention of irradiation-induced emesis. Bone Marrow Transplant 1995;15:445-8. [PubMed]
- Spitzer TR, Friedman CJ, Bushnell W, et al. Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Transplant 2000;26:203-10. [Crossref] [PubMed]
- Sicher K, Backhouse TW. An assessment of thiethylperazine (Torecan) in the control of radiation-induced nausea and vomiting. Clin Radiol 1968;19:238-40. [Crossref] [PubMed]
- Stryker JA, Demers LM, Mortel R. Prophylactic ibuprofen administration during pelvic irradiation. Int J Radiat Oncol Biol Phys 1979;5:2049-52. [Crossref] [PubMed]
- Lucraft HH, Palmer MK. Randomized clinical trial of levonantradol and chlorpromazine in the prevention of radiotherapy-induced vomiting. Clin Radiol 1982;33:621-2. [Crossref] [PubMed]
- Priestman TJ, Roberts JT, Upadhyaya BK. A prospective randomized double-blind trial comparing ondansetron versus prochlorperazine for the prevention of nausea and vomiting in patients undergoing fractionated radiotherapy. Clin Oncol (R Coll Radiol) 1993;5:358-63. [Crossref] [PubMed]
- Spitzer TR, Bryson JC, Cirenza E, et al. Randomized double-blind, placebo-controlled evaluation of oral ondansetron in the prevention of nausea and vomiting associated with fractionated total-body irradiation. J Clin Oncol 1994;12:2432-8. [Crossref] [PubMed]
- Bey P, Wilkinson PM, Resbeut M, et al. A double-blind, placebo-controlled trial of i.v. dolasetronmesilate in the prevention of radiotherapy-induced nausea and vomiting in cancer patients. Support Care Cancer 1996;4:378-83. [Crossref] [PubMed]
- Franzén L, Nyman J, Hagberg H, et al. A randomized placebo controlled study with ondansetron in patients undergoing fractionated radiotherapy. Ann Oncol 1996;7:587-92. [Crossref] [PubMed]
- Aass N, Håtun DE, Thoresen M, et al. Prophylactic use of tropisetron or metoclopramide during adjuvant abdominal radiotherapy of seminoma stage I: a randomised, open trial in 23 patients. Radiother Oncol 1997;45:125-8. [Crossref] [PubMed]
- Khoo VS, Rainford K, Horwich A, et al. The effect of antiemetics and reduced radiation fields on acute gastrointestinal morbidity of adjuvant radiotherapy in stage I seminoma of the testis: a randomized pilot study. Clin Oncol (R Coll Radiol) 1997;9:252-7. [Crossref] [PubMed]
- Sykes AJ, Kiltie AE, Stewart AL. Ondansetron versus a chlorpromazine and dexamethasone combination for the prevention of nausea and vomiting: a prospective, randomized study to assess efficacy, cost effectiveness and quality of life following single-fraction radiotherapy. Support Care Cancer 1997;5:500-3. [Crossref] [PubMed]
- Kirkbride P, Bezjak A, Pater J, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: a National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol 2000;18:1960-6. [Crossref] [PubMed]
- Lanciano R, Sherman DM, Michalski J, et al. The efficacy and safety of once-daily Kytril (granisetron hydrochloride) tablets in the prophylaxis of nausea and emesis following fractionated upper abdominal radiotherapy. Cancer Invest 2001;19:763-72. [Crossref] [PubMed]
- Martin T, Uhder K, Kurek R, et al. Does prophylactic treatment with proteolytic enzymes reduce acute toxicity of adjuvant pelvic irradiation? Result of a double-blind randomized trial. Radiother Oncol 2002;65:17-22. [Crossref] [PubMed]
- Wong RK, Paul N, Ding K, et al. 5-hydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: a placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol 2006;24:3458-64. [Crossref] [PubMed]
- Dennis K, De Angelis C, Jon F, et al. Aprepitant and granisetron for the prophylaxis of radiotherapy-induced nausea and vomiting after moderately emetogenic radiotherapy for bone metastases: a prospective pilot study. Curr Oncol 2014;21:e760-7. [Crossref] [PubMed]
- Emami H, Hematti S, Saeidian SM, et al. The efficacy of combination of ondansetron and aprepitant on preventing the radiotherapy-induced nausea and vomiting. J Res Med Sci 2015;20:329-33. [PubMed]
- Wong E, Pulenzas N, Bedard G, et al. Ondansetron rapidly dissolving film for the prophylactic treatment of radiation-induced nausea and vomiting-a pilot study. Curr Oncol 2015;22:199-210. [Crossref] [PubMed]