Patterns of care and survival outcomes of palliative radiation for prostate cancer with bone metastases: comparison of ≤5 fractions to ≥10 fractions
Introduction
Prostate cancer is one of the most common primary malignancies associated with bone metastases (1) and skeletal related events related to metastatic prostate cancer continues to create extreme economic burden in the United States (2). External radiation remains the standard of care for the palliative treatment of painful bone metastases and has an overall response rate of ranging from 78–85% (3).
Over the last several years, the focus has reducing the costs of healthcare in the United States has increased. As a result, external beam fractionation practices have come under scrutiny, especially in the palliative setting. Multiple prospective randomized studies have analyzed patients treated with either single or multi-fraction radiotherapy and have found similar pain control in the single fraction group along with significantly decreased costs (4-7). This prompted the American Society for Radiation Oncology to state that there is no evidence that longer course radiotherapy is superior to short course radiotherapy in regards to overall response rates (8).
However, the studies to date have generally included 4 Gy × 5 as long course. Though not directly comparable to bone metastases, a comparison of various palliative schedules for metastatic spinal cord compression reported that outcomes and recurrence rates of the 5 fraction regimen are more similar to the single fraction treatment than treatments consisting of ≥10 fractions (9). In the current study, we analyzed the patterns of care and survival outcomes of men with metastatic prostate cancer within the National Cancer Database (NCDB). We hypothesized that 4 Gy × 5 was underutilized in a similar fashion as single fraction radiotherapy and grouped them together to compare to longer radiation courses of ≥10 treatments.
Methods
The NCDB is a hospital-based registry that is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. It is estimated that 70% of all diagnosed malignancies in the United States are captured by facilities participating in this registry and reported to the NCDB. The Commission on Cancer’s NCDB and the hospitals participating in the NCDB are the source of the de-identified data used in this study. However, they have not verified and are not responsible for the statistical validity or conclusions derived by the authors of this study. Exemption was obtained from the New York Harbor Veterans Affairs Committee for Research and Development prior to the initiation of this study.
Men with prostate cancer metastatic to the bones diagnosed between 2004–2012 and treated with either external beam radiation were identified within the NCDB. In order to be included, men had to have been identified as receiving palliative radiation to the spine, ribs, hips, pelvic bones, shoulder, or extremity bone not otherwise specified (extremity NOS). In addition, complete data was necessary regarding the total radiation dose as well as the daily radiation fractionation schemes used. Patients were included if they received the following palliative radiation fractions: 8 Gy in one fraction, 20 Gy in 5 fractions, 30 Gy in 10 fractions, 35–37.5 Gy in 14–15 fractions, and 40–60 Gy in 20–30 fractions.
Descriptive statistics were used to analyze the patterns of care regarding the palliative radiation fractionation scheme utilized. Univariable and multivariable logistic regression were used to assess for predictors of short course treatment (defined as 1–5 fraction treatment) compared to long course (defined as 10 or more treatments). Those variables that had a p-value <0.1 on univariable analysis were included in the multivariable model. The variables analyzed included age grouping (<60, 60–70, >70 years), year of diagnosis (2004 through 2012 in single year increments), hormone therapy (yes, no), chemotherapy (yes, no), race (white, black, other), Charlson/Deyo score (0, 1, ≥2), distance from treatment center (divided into 4 quartiles), facility type (academic, non-academic), insurance type (none, private insurance, Medicaid, Medicare, government, unknown), and income level (divided into quartiles based on Census data). Overall survival (OS) was also analyzed comparing the fractionation schemes to each other and compared via the log-rank test. Multivariable Cox regression was performed utilizing the same covariables as above, with the addition of a dichotomized fractionation scheme (short course, long course). Landmark analysis was also performed on those patients surviving ≥6 months, ≥12, ≥18, and ≥24 months, with multivariable analyses performed at each landmark. Significant values were defined as those with a P value <0.05. Statistical analysis was performed using SPSS, Version 23 (IBM Inc., Armonk, NY, USA).
Results
There were 3,871 patients included in this analysis. The most common fractionation scheme was 30 Gy in 10 fractions (55.6%), followed by 37.5 Gy in 15 fractions (30.8%). The utilization of the remaining fractionation schemes consisted of 40–60 Gy in ≥20 fractions for 6.3%, 20 Gy in 5 fractions for 4.4%, and single fraction treatment for 2.9%. Patients receiving short course radiation were a median of 5 years older than those receiving long course treatments. The frequency of short course radiation increased over time from 4.1% in 2004 to 10.6% in 2012. From 2004–2008, the frequency of short course radiation was relatively stable at 3.5–5.7%. However, starting in 2009 there was an increase in the utilization of the shorter fractionation schemes to a range of 8.2–10.6%. Treatment to the ribs was associated with the highest rate of short course treatment (24.4%), while treatment of the spine was associated with the lowest rate of short course treatment (5.2%). Further details regarding patient characteristics and a comparison between groups are available in Table 1.
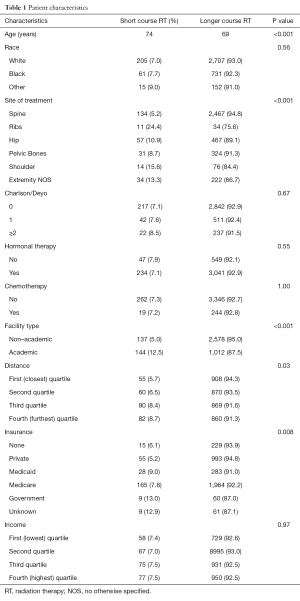
Full table
Univariable and multivariable logistic regression
On multivariable analysis, older age, years 2009-2012 (OR, 2.31–3.26), treatment at an academic center (OR, 2.93), treatment to the ribs (OR, 2.47), and those who lived further away from the treatment facility (OR, 1.48–1.59) were associated with an increased likelihood of receiving shorter fractionation. Treatment of the spine (OR, 0.34) was associated with a decreased likelihood of receiving shorter fractionation. Further details are available in Table 2.
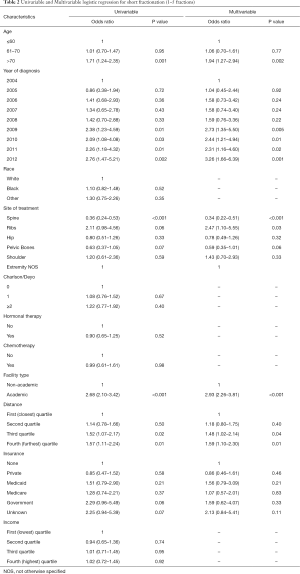
Full table
Overall survival with landmark analysis
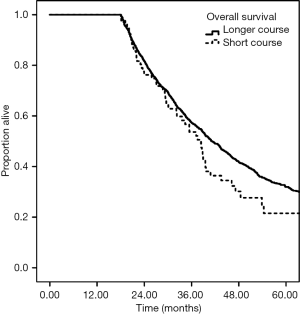
The median follow up was 19.4 months. Longer course radiation therapy was associated with increased OS at 2 years, 47.8% vs. 31.0%, P<0.001 (Figure 1). This survival difference persisted after landmark analysis for patients who survived ≥6 months, 55.2% versus 38.9%, with a hazard ratio of 0.69 (95% CI: 0.57–0.83, P<0.001) on multivariable analysis. On landmark analysis for patients who survived ≥12 months, the 2-year survival was versus 66.3% versus 51.8%, with a hazard ratio of 0.70 (95% CI: 0.56–0.88, P<0.001). However, there was no longer a survival difference on landmark analysis for patients who survived ≥18 months. The 2-year OS was 81.7% for long course versus 76.2% for short course radiation, with a hazard ratio of 0.83 (95% CI: 0.62–1.11, P=0.21) (Figure 2).

Univariable and multivariable cox regression
On univariable analysis, older age, increasing Charlson/Deyo score, receipt of chemotherapy, Medicare insurance, government insurance, and unknown insurance, were all associated with worse survival. Those who received longer fractionation schemes, hormonal therapy, or treatment to the pelvic bones were associated with improved survival. On multivariable analysis, treatment to the pelvic bones (HR, 0.73), receipt of hormonal therapy (HR, 0.81), and longer fractionation schemes (HR, 0.66) were all associated with increased survival. Increasing Charlson Deyo score (HR, 1.21–1.35), receipt of chemotherapy (HR, 1.32), age >70 (HR, 1.37), and government insurance (HR, 1.43) were associated with decreased survival. Further details are available in Table 3.
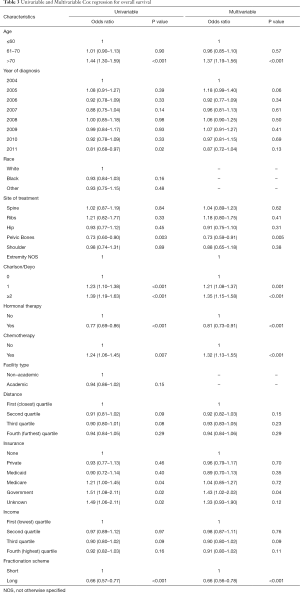
Full table
Discussion
This large hospital-based study of 3,871 men with prostate cancer metastatic to the bone revealed that the vast majority of men (91.7%) are treated with long course radiotehrapy schedules of ≥10 treatments. These findings suggest longer fractionation schemes remain more popular in the United States, despite equivalence of short radiation fractionation schemes in regards to pain control and response rates seen on prior randomized studies (4-7,10-13).
In order to reduce the cost of care, a stronger emphasis on shorter fractionation schemes may provide an avenue for future health care cost savings. Several studies have attempted to qualify this cost, with two recent studies suggesting that the current use of radiation therapy for prostate cancer bone metastases is ~$7,000–7,500 per episode of treatment (14,15). The Radiation Therapy Oncology Group (5) and the Dutch Bone Metastasis Study (16) both attempted to quantify the potential benefit of single fraction radiotherapy over 10 or more fractions. Both studies found overall lower costs with single fraction radiotherapy, even after accounting for more frequent requirement for retreatment in the single fraction arm. A SEER-Medicare based study by Bekelman et al. quantified a difference of $3,094 (95% CI: $2,107–$4,081) between single fraction and 10 or longer fraction schemes (17). Some have estimated that an absolute increase of 10% in the utilization of single fraction radiotherapy for metastatic prostate cancer would generate >70 million dollars per year in health cost savings and an increase to ~60% utilization will bring these savings to >400 million dollars annually (18,19).
The American Society for Radiation Oncology (ASTRO)issued a recent guideline statement suggesting that single fraction radiation therapy provides equivalent pain relief compared to a protracted course of radiation therapy and that no further prospective trials are necessary to confirm these findings (8). There was an increase in short course fractionation between 2009–2012 in the current study. However, the contribution of the ASTRO practice guidelines to this change and whether or not it can induce durable change are unclear. A large database-based study in Ontario reported that after an Ontario practice guideline endorsed single fraction radiotherapy use, there was a transient increase in its use for several years, followed a regression back to the pre-guideline levels (20). A second study from British Columbia suggested that a more heavy handed audit based approach is necessary to induce clinical change, though long term follow up has not yet been analyzed and it remains to be seen whether this approach is durable (18). A third study from the University of Pittsburgh demonstrated that implementation of an online clinical pathway that discouraged the use of >10 fractions and encourage single fraction radiotherapy can in fact change the practice patterns of their institution (21,22).
It should be noted that in the aforementioned studies from Ontario and British Columbia, the single fraction utilization rate was always >40% in the period 2004–2013, whereas in the present study the utilization of single or 5 fraction radiotherapy was <10%. Similarly, a Canadian national database based study identified the single fraction radiotherapy utilization rate to be 25–73% (18), whereas a prior SEER-Medicare based report encompassing patients treated from 2006–2009 reported that single fraction radiotherapy was utilized 3.3% of the time (17), consistent with the 2.9% seen in the current study. While it is unclear precisely how best to increase the utilization of single or very short fractionation courses of radiation therapy, this study clearly identifies that there is much room for improvement.
The slow uptake of shorter fractionation schemes in the United States has been seen in other subsites as well. For example, hypofractionation for breast cancer has been established as equivalent in local control and cosmesis (23), yet has seen only slow increase in utilization from 3.8% in 2006 to 13.6% in 2009 (24). There are several theories for the slow uptake of hypofractionated schedules in the United States. The first is simply that this reflects the slow cultural change away from the standard of care regimens with which physicians have been comfortable with for years. Perhaps a shift to 4 Gy × 5 would represent a compromise between the single fraction treatment with which they are not comfortable with and ≥10 or more treatments with which they are. An alternate theory is that this reflects the monetary incentives involved in healthcare in the United States, which incentivizes longer course of treatment over shorter course when found to be equivalent. Interestingly, we did note on multivariable analysis that the presence of no insurance did not result in an increased likelihood of receiving a shorter radiation scheme. This is at least suggestive that the reasons are multifactorial and not solely related to financial incentives. Additionally, the likelihood of receiving shorter fractionation is increased for those living at longer distances from their treatment centers (OR, 1.48–4.59), also suggestive of a multifactorial basis for the decision.
An additional factor likely affecting the implementation of shorter fractionation techniques in the United States is the belief that longer fractionation schemes are more durable (25). This is supported by studies that have shown that the 18–20% retreatment rate in single fraction radiotherapy is approximately double that of 10 or more fractions (26).
There are several encouraging findings from this study that suggest that there is at least an increase, albeit slow, in the utilization of short course radiotherapy. On multivariable analysis there was a relative increase in its use since 2009 (OR, 2.31–3.26). In addition, it is being used more frequently in older patients, who may have more difficulty with the daily commute or may have shorter overall life expectancies, as well as in academic centers (OR, 2.93), where physicians would be the ones expected to lead the cultural shift to shorter fractionation schemes. This latter finding is also consistent with the practice in Europe prior to the more widespread acceptance of single fraction radiotherapy (27).
We performed a landmark survival analysis in order to assess for differences in survival while accounting in some way for the likely selection bias favoring short course radiotherapy use for men with worse prognosis. Prior studies have suggested that sequential landmark analysis is an important tool to account for bias in database-based studies (28). For the present study, we performed landmark analysis in 6 month intervals in order to adjust for the likely selection bias favoring long course radiotherapy in men with better overall prognosis. We found that those receiving ≥10 fractions were associated with longer OS on multivariable analysis (HR, 0.66), which persisted on landmark analysis until OS was limited to ≥18 months. These overall survival differences are consistent with prior database based reports (17) and likely reflects the fact that patients receiving short radiation courses had overall worse prognosis in ways we are unable to measure via the NCDB. However, a recent report from the NCDB suggested that there is an OS benefit to aggressive local therapy in the metastatic setting (29). Therefore, it is also feasible that aside from selection bias, the more aggressive course of palliative radiotherapy plays a role in the improved survival seen in this study.
There are several limitations to this study inherent to the utilization of a retrospective database study. The NCDB does lacks various clinical details that may have helped guide us as to why patients received a long course radiotherapy over short course. For example, we do not know whether or not the bone metastases were complicated or uncomplicated, the number of bone metastases present or the degree of pain present. Our findings describe which fractionation schemes were used, but lack important clinical factors that may have affected the decision-making process. From the data, it is apparent that shorter fractionation schemes were utilized in men with poorer prognosis, but further insight as to the reasons why a fraction scheme was chosen would be helpful. Additionally, we do not know the efficacy of the radiation treatments, nor do we have data regarding toxicity, in order to compare regimens.
In conclusion, this large hospital-based database study in the United States reveals that men with prostate cancer metastatic to the bone are predominantly treated with palliative radiotherapy schemes of ≥10 fractions (91.7% of patients). While the use of shorter fractionation schemes has been increasing since 2010 and is used more frequently in academic settings, there is still a significant underutilization of these techniques. Increased utilization of single fraction or short fractionation schemes will likely lead to significant healthcare cost savings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Exemption was obtained from the New York Harbor Veterans Affairs Committee for Research and Development prior to the initiation of this study.
References
- Tofe AJ, Francis MD, Harvey WJ. Correlation of neoplasms with incidence and localization of skeletal metastases: An analysis of 1,355 diphosphonate bone scans. J Nucl Med 1975;16:986-9. [PubMed]
- Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics 2014;32:173-91. [Crossref] [PubMed]
- Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncology Group. Cancer 1982;50:893-9. [Crossref] [PubMed]
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804. [Crossref] [PubMed]
- Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol 2009;32:423-8. [Crossref] [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. [Crossref] [PubMed]
- 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone Pain Trial Working Party. Radiother Oncol 1999;52:111-21. [Crossref] [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol 2005;23:3366-75. [Crossref] [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594-605. [Crossref] [PubMed]
- Sze WM, Shelley MD, Held I, et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy--a systematic review of randomised trials. Clin Oncol (R Coll Radiol) 2003;15:345-52. [Crossref] [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [Crossref] [PubMed]
- Nickman NA, Ye X, Gaffney DK, et al. Cost of palliative external beam radiotherapy (EBRT) use for bone metastases secondary to prostate cancer. J Community Support Oncol 2015;13:95-103. [Crossref] [PubMed]
- Hess G, Barlev A, Chung K, et al. Cost of palliative radiation to the bone for patients with bone metastases secondary to breast or prostate cancer. Radiat Oncol 2012;7:168. [Crossref] [PubMed]
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003;95:222-9. [Crossref] [PubMed]
- Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA 2013;310:1501-2. [Crossref] [PubMed]
- Olson RA, Tiwana MS, Barnes M, et al. Use of single- versus multiple-fraction palliative radiation therapy for bone metastases: population-based analysis of 16,898 courses in a Canadian province. Int J Radiat Oncol Biol Phys 2014;89:1092-9. [Crossref] [PubMed]
- Olson RA, Tiwana M, Barnes M, et al. Impact of Using Audit Data to Improve the Evidence-Based Use of Single-Fraction Radiation Therapy for Bone Metastases in British Columbia. Int J Radiat Oncol Biol Phys 2016;94:40-7. [Crossref] [PubMed]
- Ashworth A, Kong W, Chow E, et al. Fractionation of Palliative Radiation Therapy for Bone Metastases in Ontario: Do Practice Guidelines Guide Practice? Int J Radiat Oncol Biol Phys 2016;94:31-9. [Crossref] [PubMed]
- Beriwal S, Rajagopalan MS, Flickinger JC, et al. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated National Cancer Institute-Designated Comprehensive Cancer Center Network. Int J Radiat Oncol Biol Phys 2012;83:1246-51. [Crossref] [PubMed]
- Gebhardt BJ, Rajagopalan MS, Gill BS, et al. Impact of dynamic changes to a bone metastases pathway in a large, integrated, National Cancer Institute-designated comprehensive cancer center network. Pract Radiat Oncol 2015;5:398-405. [Crossref] [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [Crossref] [PubMed]
- Jagsi R, Falchook AD, Hendrix LH, et al. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys 2014;90:1001-9. [Crossref] [PubMed]
- Hartsell WF, Konski AA, Lo SS, et al. Single fraction radiotherapy for bone metastases: clinically effective, time efficient, cost conscious and still underutilized in the United States? Clin Oncol (R Coll Radiol) 2009;21:652-4. [Crossref] [PubMed]
- Ben-Josef E, Shamsa F, Youssef E, et al. External beam radiotherapy for painful osseous metastases: pooled data dose response analysis. Int J Radiat Oncol Biol Phys 1999;45:715-9. [Crossref] [PubMed]
- Lievens Y, Kesteloot K, Rijnders A, et al. Differences in palliative radiotherapy for bone metastases within Western European countries. Radiother Oncol 2000;56:297-303. [Crossref] [PubMed]
- Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1365-73. [Crossref] [PubMed]
- Rusthoven CG, Jones BL, Flaig TW, et al. Improved Survival With Prostate Radiation in Addition to Androgen Deprivation Therapy for Men With Newly Diagnosed Metastatic Prostate Cancer. J Clin Oncol 2016;34:2835-42. [Crossref] [PubMed]


