Palliative interventions for hepatocellular carcinoma patients: analysis of the National Cancer Database
Introduction
Over the last two decades, the incidence of hepatocellular carcinoma (HCC) in the United States has increased to become the fifth most commonly diagnosed cancer and the third largest contributor to cancer-related mortality (1,2). Currently, surgical resection, liver transplantation, and ablation therapy are the only potentially curative therapies offered to patients presenting with HCC (3-6). Unfortunately, only a minority of patients are eligible for such interventions secondary to tumor (mutlifocality, size, metastasis), patient (performance status, frailty) or external (organ shortage) factors, preventing the majority of patients from receiving definitive interventions for their disease (7). Given the increased relative number of patients unable to undergo curative therapies in HCC, it is important to understand the options for patients in the non-curative paradigm.
Palliative therapies aim to alleviate symptoms, improve the quality of life, and extend survival for such patients (8,9). Palliative options for the care of patients with HCC include surgical palliation, palliative chemotherapy, regional therapies including transarterial chemoembolization (TACE), palliative radiotherapy (RT), and pain management therapies. Surgical palliation has been established for biliary decompression, which leads to fewer episodes of cholangitis and/or hepatic failure. Similarly, palliative chemotherapy (sorafenib, gemcitabine etc.) and TACE were reported to reduce mortality in HCC patients with advanced disease (10-12). Additionally, palliative RT was reported to provide pain relief from bone and adrenal metastases, and pain resulting from the enlarging tumor mass, subsequently improving patients’ survival and quality of life (11,13). While these modalities have shown variable benefits in prolonging survival, their alternative benefits in relieving symptoms allow them to be considered palliative (8-10).
Given the increasing incidence of HCC, it is important to understand the degree to which palliative therapies are clinically utilized and the survival benefit they provide. The current study aimed to compare different palliative therapies commonly utilized from a nationally representative sample of patient with HCC, using overall survival (OS) as a surrogate of efficacy. Relevant predictors of survival were also identified.
Methods
A retrospective analysis of the National Cancer Database (NCDB) was performed (1998–2011). The NCDB is a joint program of the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society (ACS), that captures approximately 70% of all invasive malignancies diagnosed at the United States (14). The study was conducted following the approval of the Institutional Review Board at the Medical College of Wisconsin.
Utilizing the liver participant user file (PUF), we queried and identified all stage I–IV HCC patients, according to the AJCC Cancer Staging Manual edition for the year of diagnosis. Subtypes of HCC were excluded. Patients that received definitive surgical resection, ablation, or liver transplantation were excluded. Patients were clustered by their disease stage according to the American Joint Committee on Cancer (AJCC) staging system, 6th edition; and patients with incomplete staging information were excluded (15). Further stratification of the cohort was performed by palliative therapy delivered. The NCDB defines palliative care as “care performed to relieve symptoms and may include surgery, radiation therapy, chemotherapy and other systemic therapies (ST) (hormone therapy, or other systemic drugs), and/or other pain management therapy” (16). Patients that did not receive any palliative therapy were also excluded from the study cohort. Clinicopathologic variables such as patient age, sex, ethnicity, Charlson comorbidity index (CCI), tumor size, disease stage, metastasis and palliative modality were collected. Treatment facilities were categorized as academic cancer centers and community cancer centers according to the COC-accreditation categories (17). Comprehensive community cancer programs were combined with community cancer programs to form the latter group.
Statistical analyses were performed using Stata 12.0 (StataCorp, College Station, TX, USA). Continuous variables were described as medians and interquartile ranges (IQR), while categorical variables were described as totals and frequencies. Data were compared by the means of Chi-squared test and Mann-Whitney test as appropriate. OS was examined by Kaplan-Meier curves and compared by log-rank test. A univariate Cox regression analysis was used to identify the factors associated with the OS, and factors that were significant on the univariate model were examined by the means of multivariate regression analysis. Hazards ratio (HR) and 95% confidence intervals were calculated for each of the variables. Alpha was set at 0.05 and a P value <0.05 was considered significant.
Results
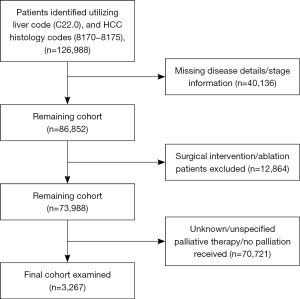
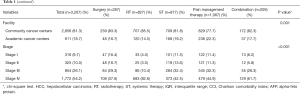
A total of 126,988 HCC cases were identified from the dataset. Of these, 3,267 met our inclusion criteria for the study period [1998–2011]; of which 287 (8.8%) received surgical palliation, 827 (25.3%) received palliative RT, 877 (26.8%) received palliative chemotherapy and other ST, 1,067 (32.6%) received pain management therapy, and 209 (6.4%) received a combination of the previous three modalities (Figure 1). The median age for the study cohort was 61 years (IQR 54–72). Overall, the majority of the study population was males (81.3%), of Caucasians ancestry (72.5%), and with no comorbidities (45.7%). A majority of the patients had a government based insurance (Medicaid/Medicare) (60.7%), and were equally treated at academic cancer centers (49.3%) vs. comprehensive community cancer centers (50.7%). Most of the study cohort presented with an advanced disease (stage IV: 54%; stage III: 26%), had an elevated alpha-feto protein (AFP) level (63%), and a tumor size ≥5 cm (75%). Information about liver cirrhosis was missing in approximately 86% of the study cohort, and was therefore excluded from further analysis. Table 1 shows the differences in the baseline clinicopathologic characteristics between palliative therapy groups.
Full table
The majority of the patients in the RT group had the lowest comorbidities (CCI 0: 57.8%), compared to the patients in the pain management therapy, which had the highest comorbidities (CCI ≥1: 64.2%; P<0.001). A majority of patients with stage IV disease received palliative RT (38.5%). The spine (35.4%) was the most commonly radiated site in the RT group, followed by the liver (18.8%) (Table 2).

Full table
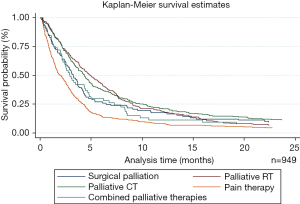
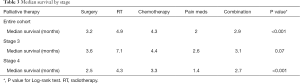
Overall, palliative RT provided the best OS, while pain management therapy provided the worst (4.9 vs. 2 m, P<0.001) (Figure 2). A similar trend was observed for patients with stage IV disease, where palliative RT provided the best OS compared to other palliative therapies (P<0.001) (Table 3). Interestingly, there was no statistically significant difference in survival between different palliative therapies for patients with stage III disease between palliative groups (P=0.07).
Full table
In a multivariate analysis, palliative RT was identified as a positive predictor of survival (HR 0.65; 95% CI, 0.50–0.83; P=0.001) (Table 4). Treatment at an academic cancer facility was also identified as a positive predictor of survival (HR 0.80; 95% CI, 0.70–0.92; P=0.002). Negative predictors of survival included older age (≥65 years) (HR 1.28; 95% CI, 1.10–1.49; P=0.001), multiple comorbidities (CCI ≥2; HR 1.34; 95% CI, 1.12–1.60; P=0.001), elevated AFP level (HR 1.61; 95% CI, 1.26–2.06; P<0.001), larger tumor size (≥5 cm) (HR 1.57; 95% CI, 1.26–2.06; P<0.001), and stage 4 disease (HR 1.37; 95% CI, 1.23–2.00; P<0.001). Pain management therapy was also identified as a negative predictor of survival (HR 1.54; 95% CI, 1.20–1.96; P<0.001).

Full table
Discussion
The alarming rise in HCC incidence and mortality over the past few decades in the US and the limited curative interventions has subsequently led to the investigation of alternative therapies. Palliative therapies provided promising outcomes for such patients, varying between better quality of life, symptom control and a potential improvement in survival. Recent studies suggest that early palliative care improves the quality of life and mood in addition to prolonging survival (18). More specifically, previous studies have shown benefit with palliative therapies in the care of patients with unresectable HCC. Davila et al report a reduction in mortality following the receipt of TACE or systemic chemotherapy in HCC patients not eligible to receive definitive management options (19). Others have shown improved survival provided by palliative RT for patients with spinal metastasis from HCC (13,20). Few studies compared these different palliative modalities to assist in determining the best approaches in the palliative management of HCC (8). The current study sought to examine and compare different palliative therapies offered to patients with HCC by assessing their impact on the OS. The results suggest that palliative RT provides the best survival outcomes for patients with HCC; most noticeable in patients with stage IV disease; followed by palliative chemotherapy and other ST; whereas pain management therapy provided the worst survival outcomes (P<0.001).
There is a burgeoning and established literature that suggest the benefit of palliative RT in providing adequate control of HCC mass, relieving symptoms, improving the quality of life and extending survival in HCC and other unresectable malignancies (13,21-24). As it pertains to quality of life indices, Hawkins and Dawson reported improvement of symptoms in HCC patients presenting with lymph node metastases, brain metastases and bone metastases following the receipt of palliative RT (22). Similarly, Hayashi et al. emphasized the importance of palliative RT in relieving pain and improving the quality of life in HCC patients presenting with bone metastasis, a form which is unique to HCC which causes both bone and neuropathic pain (13). Other reports show that palliative RT leads to the reduction of tumor mass effects and pain from bulky disease, in addition to the cessation of bleeding and the prevention of tumor rupture (22). When evaluating survival, recent studies have shown stereotactic beam radiotherapy (SBRT) to be at least equivalent to radiofrequency ablation (RFA) and potentially a curative treatment in early stage HCC (25-27). While there is currently no phase III data to support SBRT alone, these findings and the growing body of literature supports evaluation with further studies to elucidate the optimal timing, means of administration (external beam radiation therapy, stereotactic beam RT, etc.), and radiation dose required to deliver the utmost benefit of palliative RT. This is important given our findings of significance in the multivariate analysis as compared to univariate analysis for radiation therapy. Given that stage adjustment made a difference it will be key to look at a more robust database or those with more patient’s to case match, allowing for more definitive conclusion.
Similar to previous studies, the current study identified AFP level, tumor size, and comorbidities as predictors of survival (28,29). In our analysis we have also demonstrated the impact of treating facility type on survival, where the treatment at academic cancer centers was identified as a positive predictor of survival compared to treatment at community cancer centers. The difference seen might be explained by the variability in the quality of care and expertise level between different cancer center categories. Academic centers cared for significantly more patients with advanced stage HCC or those who were high risk for operative intervention. In addition, comprehensive community cancer centers had worse survival when compared to academic centers. These findings are similar to other studies which demonstrate the disparate effect location of cancer care can have on treatment decision and outcomes (30). This finding is seen even when considering therapeutic care for HCC nationally (31-33). Our data then suggest that we have a disparity across the continuum of care for HCC in the US. Given the substantial resources and complexity in management of HCC it is possible that academic hospitals may be better suited. Further, research is then needed to determine best practice in the palliative management of patients with HCC. The results of the current study, however, fail to identify insurance status as a predictor of survival. This finding is important as previous reports show that insurance status might impact the survival of patients presenting with HCC (34). This suggests that as government insurance is expanded through the Affordable Care Act patients will receive equivalent care to other cohorts with private insurance. An alternative view point would suggest that the outcomes of patients in this group are universally poor.
The current study has several limitations. Similar to other administrative datasets, the NCDB might contain inadequately populated information regarding disease status, HCC etiology and treatment details. For example, the absence of cirrhosis data in approximately 86% of patients may skew findings as this population represents a larger proportion of HCC patients and the assessment of the impact of palliative care consultation on cirrhotic versus non-cirrhotic would be useful. Despite this, the findings suggest an area in need of further study and open the door for future investigation. Patients’ quality of life is not recorded in this dataset, which limited the outcomes examined to OS. While this outcome might not accurately compare the efficacy of different palliative modalities, it can represent a good surrogate of the overall efficacy of different palliative modalities in patients with late stage disease, where the majority of patients have a limited life expectancy. The NCDB database does not make distinction on curability. Some physicians may consider this an issue however, given the fact that patients received palliative therapies this suggest that regardless of stage of disease there was potentially a clinical scenario which prohibited resection, opening the door for palliative therapies. Additional bias might have resulted from the grouping of patients receiving chemotherapy with those receiving hormonal therapies and other systemic drugs, which can potentially censor any additional survival benefit provided by any of the various regimens that are utilized. Finally, it is important to realize that this analysis was not able to assess the clinical factors that impacted the receipt of different palliative therapies. The choice of a certain palliative therapy might have been a result of the patients’ performance status, where the patients who are able to withstand aggressive treatments received RT or CT, while those with worse prognosis and/or vital status received pain medicine only for palliation. Studies examining these factors are still warranted in the future.
Conclusions
The current study suggests that palliative RT provides the best OS amongst all palliative therapies delivered to HCC patients. Disease stage, tumor size, treating facility, AFP level, comorbidities, and age are all predictors of survival which must be considered in the setting of palliation. Given the previous reports showing the importance of palliative RT in improving the quality of life, controlling local disease and extending survival, and based on the results of this study, palliative RT should be considered in a multidisciplinary fashion for patients presenting with stage IV disease. This manuscript establishes a roadmap for further research to improve the care of the substantial populations of patients who are ineligible for curative therapies for HCC. Future research is needed to evaluate the best practice for RT as well as combination therapy in the provision of care. This would include the evaluation of quality of life indices and economic analysis in comparison of the effect of different palliative therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817-23. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- Zhou J, Wang Z, Qiu SJ, et al. Surgical treatment for early hepatocellular carcinoma: comparison of resection and liver transplantation. J Cancer Res Clin Oncol 2010;136:1453-60. [Crossref] [PubMed]
- Burak KW, Bathe OF. Is surgical resection still the treatment of choice for early hepatocellular carcinoma? J Surg Oncol 2011;104:1-2. [Crossref] [PubMed]
- Miura JT, Johnston FM, Tsai S, et al. Surgical resection versus ablation for hepatocellular carcinoma ≤ 3 cm: a population-based analysis. HPB (Oxford) 2015;17:896-901. [Crossref] [PubMed]
- Groeschl RT, Gamblin TC, Turaga KK. Ablation for hepatocellular carcinoma: validating the 3-cm breakpoint. Ann Surg Oncol 2013;20:3591-5. [Crossref] [PubMed]
- Cooper A, Aloia T. Surgical resection for hepatocellular carcinoma. Transl Cancer Res 2013;2:450-59.
- Amini A, Gamblin TC. Palliation: treating patients with inoperable biliary tract and primary liver tumors. Surg Oncol Clin N Am 2014;23:383-97. [Crossref] [PubMed]
- Cunningham SC, Choti MA, Bellavance EC, et al. Palliation of hepatic tumors. Surg Oncol 2007;16:277-91. [Crossref] [PubMed]
- Kumar M, Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol 2014;4:S130-9. [Crossref] [PubMed]
- Zeng ZC, Tang ZY, Fan J, et al. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol 2005;35:61-7. [Crossref] [PubMed]
- Meza-Junco J, Montano-Loza AJ, Liu DM, et al. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev 2012;38:54-62. [Crossref] [PubMed]
- Hayashi S, Tanaka H, Hoshi H. Palliative external-beam radiotherapy for bone metastases from hepatocellular carcinoma. World J Hepatol 2014;6:923-9. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Greene FL, Fleming ID, et al. editors. AJCC Cancer Staging Manual. Philadelphia: Lippincott-Raven, 2002:131-6.
- National Cancer Database Data Dictionary, 2013. Available online: http://ncdbpuf.facs.org/?q=print-pdf-all
- CoC Accreditation Categories: American College of Surgeons. Available online: https://www.facs.org/quality-programs/cancer/accredited/about/categories
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Davila JA, Duan Z, McGlynn KA, et al. Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States. J Clin Gastroenterol 2012;46:71-7. [Crossref] [PubMed]
- Nakamura N, Igaki H, Yamashita H, et al. A retrospective study of radiotherapy for spinal bone metastases from hepatocellular carcinoma (HCC). Jpn J Clin Oncol 2007;37:38-43. [Crossref] [PubMed]
- Hoffe SE, Finkelstein SE, Russell MS, et al. Nonsurgical options for hepatocellular carcinoma: evolving role of external beam radiotherapy. Cancer Control 2010;17:100-10. [PubMed]
- Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer 2006;106:1653-63. [Crossref] [PubMed]
- McDonald R, Chow E, Rowbottom L, et al. Quality of life after palliative radiotherapy in bone metastases: A literature review. J Bone Oncol 2014;4:24-31. [Crossref] [PubMed]
- Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-6. [Crossref] [PubMed]
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. [Crossref] [PubMed]
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399-404. [Crossref] [PubMed]
- Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One 2013;8:e79854. [Crossref] [PubMed]
- Nathan H, Schulick RD, Choti MA, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg 2009;249:799-805. [Crossref] [PubMed]
- Zhang Q, Shang L, Zang Y, et al. α-Fetoprotein is a potential survival predictor in hepatocellular carcinoma patients with hepatitis B selected for liver transplantation. Eur J Gastroenterol Hepatol 2014;26:544-52. [Crossref] [PubMed]
- Hyder O, Dodson RM, Nathan H, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J Am Coll Surg 2013;217:896-906. [Crossref] [PubMed]
- Hoehn RS, Hanseman DJ, Jernigan PL, et al. Disparities in care for patients with curable hepatocellular carcinoma. HPB (Oxford) 2015;17:747-52. [Crossref] [PubMed]
- Shah SA, Smith JK, Li Y, et al. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer 2011;117:1019-26. [Crossref] [PubMed]
- Nathan H, Segev DL, Bridges JF, et al. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol 2013;20:448-56. [Crossref] [PubMed]
- Zaydfudim V, Whiteside MA, Griffin MR, et al. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann Surg Oncol 2010;17:3104-11. [Crossref] [PubMed]