Palliative care units in lung cancer in the real-world setting: a single institution’s experience and its implications
Introduction
Lung cancer is the leading cause of cancer-related deaths in the world and is associated with substantial symptom burden affecting physical, psychological and social aspects of life (1,2). Compared to patients with other malignancies, lung cancer patients have the greatest number of symptoms and highest prevalence of both psychological distress and existential concerns (2,3). When referred to palliative care units, pain, dyspnea and anorexia are among the most prevalent and severe ones (4). Lung cancer patients and their family caregivers are those with the highest number of unmet needs in psychological, physical and daily-activity concerns compared to all other cancer patients (5,6). Palliative care units have recently been established at major cancer centers. These units primarily focus on anticipating, preventing, diagnosing, and treating symptoms experienced by patients with serious or life-threatening illnesses helping them and their families to make medically important decisions (7). They also should provide access of cancer patients to experts specifically trained in palliative care. Palliative care has traditionally been delivered late during the course of disease in patients with cancer, usually at the time of uncontrolled symptoms (8). Integration of early palliative care in the overall management of patients with advanced lung cancer was recently shown to lead to clinically meaningful improvement in quality of life, less aggressive end-of-life care and potentially prolonged survival (9-12). Therefore, it has been suggested to be offered earlier in the course of disease than according to current practice (9,10,13). Here we summarize our experience on the role of the palliative care unit at the Medical University of Vienna in the management of patients with advanced lung cancer. We report symptoms leading to admission, interventions during hospitalization and based on these findings we suggest strategies for future improvement in the palliative care of patients with advanced lung cancer.
Methods
Patients
The data of all patients with lung cancer treated at the palliative care unit between June 2010 and March 2013 were retrospectively reviewed. We assessed the following patient characteristics: age, gender, Karnofsky performance status scale, body mass index (BMI), histology and tumor stage. Reasons for admission, treatment as well as interventions during hospitalization, and outcomes were determined. The study was approved by the local ethics committee of the Medical University of Vienna, Austria (1386/2014).
Statistical analysis
Descriptive statistics were used to characterize clinical and demographic parameters. Survival times were estimated from diagnosis until death or last-follow up by means of Kaplan-Meier analysis. Data were analysed using IBM® SPSS version 20.
Results
Patient characteristics
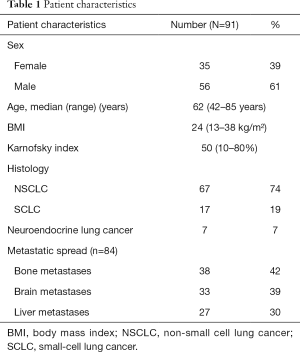
Between June 2010 and March 2013, 460 patients were treated at our palliative care unit. Ninety-one (19.8%) patients suffered from lung cancer, comprising the largest group of cancer patients. The characteristics of these patients are summarized in Table 1: 39% (35/91) females, 61% (56/91) males, age median 62 years (range, 42–85 years), BMI median 24 (range, 13–38), Karnofsky performance status scale median 50% (range, 10–80%); 74% (67/91) non-small cell lung cancer (NSCLC), 19% (17/91) small-cell lung cancer (SCLC), 7% (7/91) neuroendocrine lung cancer, 92% had metastatic disease. Bone metastases were present in 42% (38/91), brain metastases in 36% (33/91), and liver metastases in 30% (27/91) of the patients. Metastases in more than two organ systems were present in 34% (31/91) of the patients.
Full table
Primary reasons for admission to the palliative care unit
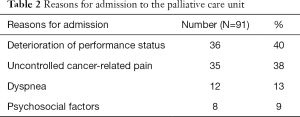
Although reasons for admission could be several-fold, main reasons for admission of patients were deterioration of performance status in 40% (36/91), uncontrolled cancer-related pain in 38% (35/91), dyspnea in 13% (12/91), and psychosocial factors in 8% (8/91) of the patients (Table 2).
Full table
Palliative treatment during hospitalization
Treatment focused on symptom management, psychosocial support and, whenever considered to be beneficial, on anti-tumor therapy. Further care included dietary counselling and physiotherapy (mobilization and muscle strengthening, breathing strategies). Psychosocial support was delivered by a psychologist or psychotherapist. Access to a chaplain, a social worker and volunteers were also offered to patients. Deterioration of performance status as the main reason for admission was treated by a multidisciplinary approach consisting of symptom management, dietary counselling, enteral or parenteral nutrition in selected patients, physiotherapy and psychosocial support. Analgesic treatment according to step I–III of the WHO ladder was the most common treatment modality and was delivered to 84% (76/91) of the patients. Opioid medication was initiated in 24% (22/91) of the patients and was continued including dose-modification when indicated. Rapid acting opioids to control refractory pain and/or dyspnea were delivered to 75% (68/91) of the patients. To treat cancer-related intractable pain, implantation of an intrathecal pain pump and brachial plexus neurolysis were performed in one patient each. Dyspnea was also common and caused by cancer-related disease progression resulting in pleural effusions or airway obstructions. Pleural effusions were treated by thoracocentesis (n=4), implantation of PleurX drainage systems (n=3) and chemical pleurodesis (n=1). Airway obstruction was re-solved by stenting in two patients. Stents were also applied to the vena cava superior and the oesophagus. Rare interventions included peritoneocentesis of malignant ascites (n=1), haemodialysis due to acute renal failure (n=1) and endoscopic retrograde cholangiopancreatography and stenting (n=1). Palliative chemotherapy was administered in 8% (7/91) and antibiotic therapy was delivered to 33% (30/91) of the patients. Eighteen percent (16/91) of the patients received palliative radiotherapy. Radiotherapy included bone radiation (n=10), mediastinal radiation (n=3) and whole brain radiation (n=3). Psychosocial impairment was treated by a psychologist and psychotherapist. In addition, 27% (25/91) of the patients received antidepressants or other psychopharmacological medication. Most patients had late stage disease with refractory symptoms in terms of pain, dyspnea and delirium. They required end-of-life care. Palliative sedation by means of a continuous intravenous or subcutaneous infusion with midazolam was administered in 24% of the patients.
Clinical outcome
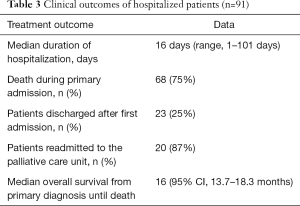
The clinical outcome of patients is summarized in Table 3. Median duration of hospitalization at the palliative care unit was 16 days (range, 1–101 days). Twenty-five percent of the patients had improvement or stabilisation of tumor-related symptoms at the time of discharge. Median Karnofsky performance status scale was 60% (50–70%). Sixteen percent of the patients showed improvement in their Karnofsky performance status scale. Seventy-five percent (68/91) of the patients died during their first admission to the palliative care unit due to late-stage lung cancer. Readmission occurred in 20 out of 23 patients. Eight patients were admitted twice, 7 patients three times and one patient was admitted a fourth and one a fifth time. Median survival from primary diagnosis of lung cancer until death was 16 months (95% confidence interval, 13.7–18.3 months).
Full table
Discussion
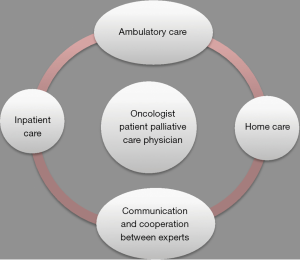
Lung cancer comprised the single most frequent cancer type among patients treated at our palliative care unit. Reasons for admission of patients were cancer-related symptoms requiring inpatient care. The majority of the patients with lung cancer had late-stage disease which explains the high rate of deaths during hospitalization. Thus, this may also reflect that oncologists consider palliative care units mainly as hospices. However, recent data suggest benefit from involvement of palliative care earlier during the course of disease (9,12). Approximately 80% of all lung cancer patients suffer from multiple physical and psychological symptoms (2-4). To properly deal with these complex multidimensional symptoms, patients at the palliative care unit had access to a multidisciplinary team involving cancer specialists, physiotherapists, dieticians, social workers, psychologists and psychotherapists, chaplains and passionate volunteers is crucial (14). Access to these specialists has been offered to all our patients and usually has been well accepted depending on the overall situation of the patients. This multidisciplinary approach is a major component of palliative care units, allowing to address physical, emotional, spiritual and social concerns. Cancer-related deterioration of performance status was the most common reason for admission and was often associated with other cancer-related symptoms including cachexia, dyspnea, pain and psychosocial impairment. Insufficient control of cancer-related pain was another important reason for admission. Cancer pain can be caused by nociceptive, neuropathic or mixed pathogenic mechanisms and is affected by anxiety, depression or spiritual fears (15). Neuropathic pain is particularly common in patients with lung cancer and can be caused by nerval injury, chemotherapy-induced peripheral neuropathy or other factors (16,17). Pain management is summarized in Table 4. Opioids play a major role in treatment. Other treatment options include anticonvulsants (e.g., pregabalin and gabapentin), antidepressants (e.g., duloxetine and tricyclics), and topical treatments (e.g., capsaicin and lidocaine) (17). In 10–20% of all patients, adequate pain control cannot be achieved by drugs. In these patients, interventional pain management techniques such as epidural and intrathecal infusion therapies or neurolytic interventions may be helpful (22). Pancoast tumors may invade the brachial plexus, thereby producing severe neuropathic pain (23). Since opioids often fail in these situations, local nerve blockades remain an important therapeutic option (23,24). Palliative radiation is another relevant treatment option in patients with lung cancer (25). Particularly in case of bone or brain metastases a single fraction of radiotherapy for palliation of painful bone metastases may be preferable because it is less toxic, more convenient and appears to be as efficacious as prolonged treatment schedules (25). Dyspnea occurs in patients with late-stage lung cancer. Its mechanisms are complex and include airway obstructions, pleural-effusions and pulmonary metastases (26). Management of dyspnea is outlined in Table 5. Treatment of dyspnea requires special expertise of the treating physicians (31). With regard to management of malignant pleural effusions, thoracocentesis is recommended for patients with a short life expectancy and pleurodesis with intrapleural drug instillation for those with a longer life expectancy. Implantation of an indwelling pleural catheter is an option for patients who are unsuitable for chemical pleurodesis (26,32). These catheters allow patients to drain their pleural effusion by themselves, thereby offering them more self-dependence and comfort (33). In the case of obstruction of airways or superior vena cava, stent insertions may provide relief. The proper selection of these intervention requires a close cooperation among multidisciplinary teams including pulmonologists, interventional radiologists and thoracic surgeons (34). Dietary counselling and nutrition support was offered to every patient although its clinical impact remains to be proven in randomized trials (35). Generally, deterioration of performance status often involves weight loss. Our patient cohort had a normal weight with an average BMI of 24. However, a normal BMI might lead to underdiagnose the clinically relevant symptom of sarcopenia in patients suffering from lung cancer (36). Management of cancer-related cachexia is summarized in Table 6. In order to allow discharge of patients from hospital and avoid readmissions, the organization of an appropriate home environment including support by a social worker is important. Furthermore, mobile palliative care teams have been organized for all discharged patients and were usually well accepted by the patients because most patients would prefer to die at home (7). Palliative care is associated with an increase in symptom control medications and decrease in medications for co-morbid conditions, while polypharmacy still is a problem (41,42). Future perspectives consist of optimizing care by connecting ambulatory care, inpatient care and home care. This care should involve oncologists and palliative care specialists. Team discussions in form of communication and cooperation between experts may also be of help (Figure 1). Based on our experience, patients with advanced lung cancer represent the largest entity of patients at our palliative care unit. Even though the majority of the patients died at the palliative care unit due to late-stage disease, patients had improvement or stabilisation of tumor-related symptoms and did benefit from their admission. The high symptom burden of metastatic lung cancer requires early integration of palliative care into standard oncology treatment. Symptom control regarding pain, dyspnea, weight loss and mental health are most important. Our study revealed late admission as an important shortcoming with regard to advanced lung cancer. Possible reasons are that patients prefer to stay at home as long as possible. The high percentage of deaths during primary admission to our palliative care unit indicated that patients with lung cancer were mainly admitted to palliative care for end-of-life care. This practice also suggests that palliative care units are still widely misconceived as hospices. Education and information of physicians and patients on the role of palliative care units and their role in the overall management of patients with lung cancer are warranted. In order for the large number of patients with advanced diseases to benefit from palliative care it is important that palliative care is incorporated into the practices of all clinicians caring for persons with serious and complex illnesses. Training of medical oncologists on palliative care is important. Routine integration of palliative care physicians into tumor boards is another important step. Finally, promotion of ambulant and outpatient palliative care consultations by mobile palliative care teams will allow patients to stay within their homes as long as possible, where the majority of patients would prefer to die. Palliative care units have contributed to inpatient care of patients with advanced lung cancer. Further focus on the original intent of palliative care units might result in even greater importance of these units. Palliative care interventions should become a matter of course in the treatment of patients suffering from lung cancer. The expertise of both oncologists and palliative physicians should lead to an optimal treatment strategy.

Full table

Full table

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee of the Medical University of Vienna, Austria (No. 1386/2014).
References
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii56-64. [Crossref] [PubMed]
- Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer 1995;71:633-6. [Crossref] [PubMed]
- Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol 2000;18:893-903. [PubMed]
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001;4:157-65. [Crossref] [PubMed]
- Girgis A, Lambert SD, McElduff P, et al. Some things change, some things stay the same: a longitudinal analysis of cancer caregivers' unmet supportive care needs. Psycho-oncology 2013;22:1557-64. [Crossref] [PubMed]
- Liao YC, Liao WY, Shun SC, et al. Symptoms, psychological distress, and supportive care needs in lung cancer patients. Support Care Cancer 2011;19:1743-51. [Crossref] [PubMed]
- Rome RB, Luminais HH, Bourgeois DA, et al. The role of palliative care at the end of life. Ochsner J 2011;11:348-52. [PubMed]
- Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009;27:206-13. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Parikh RB, Kirch RA, Smith TJ, et al. Early specialty palliative care--translating data in oncology into practice. N Engl J Med 2013;369:2347-51. [Crossref] [PubMed]
- Tokito T, Murakami H, Mori K, et al. Implementation status and explanatory analysis of early advance care planning for Stage IV non-small cell lung cancer patients. Jpn J Clin Oncol 2015;45:261-6. [Crossref] [PubMed]
- Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 2015;33:1438-45. [Crossref] [PubMed]
- Bauman JR, Temel JS. The integration of early palliative care with oncology care: the time has come for a new tradition. J Natl Compr Canc Netw 2014;12:1763-71. [PubMed]
- Otis-Green S, Sidhu RK, Del Ferraro C, et al. Integrating social work into palliative care for lung cancer patients and families: a multidimensional approach. J Psychosoc Oncol 2014;32:431-46. [Crossref] [PubMed]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49. [Crossref] [PubMed]
- Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420-33. [Crossref] [PubMed]
- Fallon MT. Neuropathic pain in cancer. Br J Anaesth 2013;111:105-11. [Crossref] [PubMed]
- Higginson IJ, Gao W. Opioid prescribing for cancer pain during the last 3 months of life: associated factors and 9-year trends in a nationwide United Kingdom cohort study. J Clin Oncol 2012;30:4373-9. [Crossref] [PubMed]
- Pargeon KL, Hailey BJ. Barriers to effective cancer pain management: a review of the literature. J Pain Symptom Manage 1999;18:358-68. [Crossref] [PubMed]
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58-68. [Crossref] [PubMed]
- Bruera E, Paice JA. Cancer pain management: safe and effective use of opioids. Am Soc Clin Oncol Educ Book 2015.e593-9. [Crossref] [PubMed]
- McHugh ME, Miller-Saultz D, Wuhrman E, et al. Interventional pain management in the palliative care patient. Int J Palliat Nurs 2012;18:426-8, 430-3. [Crossref] [PubMed]
- Gofeld M, Bhatia A. Alleviation of Pancoast's tumor pain by ultrasound-guided percutaneous ablation of cervical nerve roots. Pain Pract 2008;8:314-9. [Crossref] [PubMed]
- Peláez R, Pascual G, Aguilar JL, et al. Paravertebral cervical nerve block in a patient suffering from a Pancoast tumor. Pain Med 2010;11:1799-802. [Crossref] [PubMed]
- Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013;119:888-96. [Crossref] [PubMed]
- Bertolaccini L, Zamprogna C, Barberis L, et al. Malignant pleural effusions: review of treatment and our experience. Rev Recent Clin Trials 2007;2:21-5. [Crossref] [PubMed]
- Lanken PN, Terry PB, Delisser HM, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912-27. [Crossref] [PubMed]
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435-52. [Crossref] [PubMed]
- Mahler DA, Selecky PA, Harrod CG, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137:674-91. [Crossref] [PubMed]
- Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008;148:141-6. [Crossref] [PubMed]
- Max Watson CL, Hoy A, Wells J. editors. Oxford Handbook of Palliative Care. Second Edition. Oxford: Oxford University Press, 2009.
- Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol 2009;35:546-51. [Crossref] [PubMed]
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, et al. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncologica 2012;51:996-1008. [Crossref] [PubMed]
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475-84. [Crossref] [PubMed]
- Rueda JR, Sola I, Pascual A, et al. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Syst Rev 2011.CD004282. [PubMed]
- Kovarik M, Hronek M, Zadak Z. Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer 2014;84:1-6. [Crossref] [PubMed]
- Del Fabbro E, Hui D, Dalal S, et al. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med 2011;14:1004-8. [Crossref] [PubMed]
- Argilés JM, Busquets S, Stemmler B, et al. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 2015;22:100-6. [Crossref] [PubMed]
- Amano K, Maeda I, Morita T, et al. Need for nutritional support, eating-related distress and experience of terminally ill patients with cancer: a survey in an inpatient hospice. BMJ Support Palliat Care 2016;6:373-6. [Crossref] [PubMed]
- Kotler DP. Cachexia. Ann Intern Med 2000;133:622-34. [Crossref] [PubMed]
- Hui D, Li Z, Chisholm GB, et al. Changes in medication profile among patients with advanced cancer admitted to an acute palliative care unit. Support Care Cancer 2015;23:427-32. [Crossref] [PubMed]
- Kierner KA, Weixler D, Masel EK, et al. Watzke HH. Polypharmacy in the terminal stage of cancer. Supp Care Cancer 2015;5:5.