Radiation therapy for the treatment of skin Kaposi sarcoma
Introduction
Kaposi sarcoma (KS) is a non-curable malignancy caused by infection with human herpes virus 8 (HHV8) which can present as skin lesion with or without internal organ involvement (1,2).
KS lesions are purplish, reddish blue or dark brown/black macules, plaques or nodules which involve the skin and mucous membranes. These lesions may bleed, ulcerate and may be associated with lymphedema, pain and secondary infection (3). Most patients with KS have a long indolent chronic course.
There are four subtypes of KS (4):
- Classical KS affecting those of Mediterranean descent or Eastern European descent;
- African endemic KS;
- Iatrogenic immunosuppressed KS;
- AIDS (epidemic) related KS.
Localized cutaneous KS may be treated with radiotherapy, cryotherapy, intralesional injections of vinblastine or topical immunotherapy. Extensive skin or internal involvement with KS may be treated with chemotherapy or immunotherapy. Highly active antiretroviral therapy (HAART) is considered first-line therapy for AIDS related KS. For iatrogenic KS due to immunosuppression, reduction or discontinuation of immunosuppressive therapy is recommended (5).
Radiation therapy is used to help regress KS skin lesions causing local symptoms such as bleeding or pain (6).
Methods
A retrospective review was undertaken for all KS patients treated with radiotherapy at a tertiary cancer centre from Jan. 2, 1999 to Dec. 31, 2014 (inclusive). This study was approved by the local hospital Research Ethics Board.
Demographic information (date of birth, sex, co-morbidities) were retrieved, radiotherapy treatment details, symptom and side-effects outcomes were recorded.
Complete response (CR) was defined as complete visual disappearance. Partial response (PR) was defined as 50% or more regression and progressive disease defined as growth of the lesion.
Results
A total of 47 patients with KS (43 classical, 0 African, 1 iatrogenic, 3 AIDS related) were seen in the multidisciplinary clinic. Nineteen patients with asymptomatic cutaneous skin KS were observed. Four patients were treated with surgery. Three patients were treated with cryotherapy and two patients were treated with electrodessication and curettage. One patient was treated with Aldara cream. One patient had iatrogenic KS due to immunosuppressive drugs. With the withdrawal of the immunosuppressive, the KS lesions regressed.
This left a total of 17 patients (5 females and 12 males) with 97 KS skin sites treated with local external beam radiotherapy (Table 1). There were an additional 18 skin sites which were treated with repeat radiotherapy (Table 2). The subtypes of KS treated with radiotherapy were 14 classical, 0 African, 0 iatrogenic, 3 AIDS related. Ages at the time of initial radiotherapy ranged from 44–93 years of age. The radiotherapy dose ranged from 6 Gy in 1 fraction to 30 Gy in 10 fractions, with the most common dose fractionation scheme being 8 Gy in 1 fraction or 20 Gy in 5 daily fractions. The number of KS sites treated with radiotherapy per patient ranged from 1–22 sites (including repeat radiotherapy sites). The median follow-up time was 26.1 months (range, 0.3 months to 28.5 years).
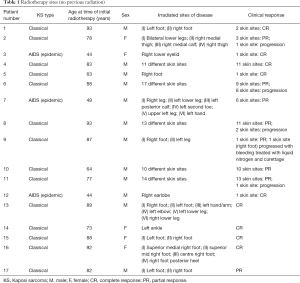
Full table

Full table
For 97 previously untreated KS sites, 87% responded to radiation (30% CR and 57% PR). Thirteen percent of KS sites treated with radiation progressed.
Of the 13% (n=13) KS skin sites which progressed despite radiation, all were treated with repeat radiotherapy except one was treated with liquid nitrogen. There were a total of 18 repeat radiotherapy fields (as some of the originally treated fields were broken up into smaller fields). The CR, PR and progression rates for repeat radiotherapy KS sites were 0%, 50% and 50% respectively. One patient with two skin sites treated with repeat radiation was lost to follow-up.
No fatal toxicities occurred. The most common side effects were dry desquamation, hyperpigmentation and lymphedema.
Discussion
KS was first described in 1872 by Moritz Kaposi. Microscopic characteristics include disorganized endothelial cell proliferation and blood-filled vascular clefts with areas of organized micro-neovascularizations. An inflammatory infiltrate is also commonly seen (1). Since the description of KS (a classical or Mediterranean type) by Moritz Kaposi, two additional forms were identified (African endemic KS and post-transplantation or iatrogenic KS). With the emergence of the AIDS epidemic in the early 1980s, the 4th type AIDS (epidemic) emerged.
In this present series, the most common KS type was classical. While epidemiologic studies indicate that the incidence of classic KS has remained stable over the years, the incidence of AIDS related KS began to fall in the late 1980s with the use of HIV directed treatments. Subsequently with the use of HAART, which partially restores the immune system, the incidence of AIDS related KS has dropped dramatically (7). Our radiation series did not include any patient with iatrogenic/post-transplantation KS. The incidence of KS after transplantation is low at 8.8 per 100,000 person years among transplant recipients in North America (8).
A systematic review published in 2012 regarding the management for classic KS was undertaken by Régnier-Rosencher et al. (6). There were only 26 articles with at least five patients included in the systematic review. Of these included studies, only four were radiotherapy publications (9-12). The number of patients included in these radiotherapy series ranged from 27 patients to 209 patients. All the series were retrospective except one (9).
The Yildiz et al. publication (9) was a prospective non-randomized study of 8 Gy given prior to 1998 and then 6 Gy given in 1998 to 2004. The authors reported that CR at 12 months was 93% with 8 Gy and 60% with 6 Gy, P<0.0001. It was concluded that a single 8 Gy is more effective for CR as compared to a single 6 Gy. From the four included radiotherapy studies (9-12), the CR plus PR rates ranged from 85–99.5%.
There is a lack of controlled prospective studies which examine the optimal radiation dose fractionation schedule for response and symptom control. Reported dose fractionation schedules range from 6 Gy in 1 fraction to radical dose fractionation schemes to a total of 45 Gy (9). A randomized trial was reported for AIDS associated KS. Sixty patients with 65 sites of AIDS related KS skin sites were randomized to 24 Gy in 12 fractions versus 20 Gy in 5 fractions. The authors concluded that response and local control rates were not statistically different between the two arms. Acute and late skin toxicity were also not statistically different between the two arms (13).
Our series of patients had similar high response rates (87% CR and PR) after radiation as compared to the published literature. In addition to the existing literature, we were able to report on a subset of patients treated with repeat radiotherapy for KS skin lesions which failed initial radiotherapy. In this subgroup, none of the patient had CR, half showed PR and half showed continued progression.
The limitations of this study include the retrospective nature of the analysis, the lack of validated quality of life and symptom control outcomes. In addition various radiotherapy dose fractionation schedules were used.
Conclusions
In this cohort of patients, the majority of KS skin lesions (87%) responded to radiotherapy. Patients experience minimal side effects from the palliative radiation regimens used. KS skin lesions which progress despite radiation are unlikely to show CR with repeat radiotherapy. In our experience 50% of repeat radiotherapy KS skin lesions will have partial regression and 50% will have continued progression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of Sunnybrook Health Sciences Centre (REB project identification number 464-2014) and written informed consent was obtained from all patients.
References
- Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med 1995;332:1181-5. [Crossref] [PubMed]
- Gramolelli S, Schulz TF. The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma. J Pathol. 2015;235:368-80. [Crossref] [PubMed]
- Bhutani M, Polizzotto MN, Uldrick TS, et al. Kaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatment. Semin Oncol 2015;42:223-46. [Crossref] [PubMed]
- National Cancer Institute. Kaposi Sarcoma Treatment (PDQ®)–Health Professional Version. Available online: http://www.cancer.gov/types/soft-tissue-sarcoma/hp/kaposi-treatment-pdq
- Ruocco E, Ruocco V, Tornesello ML, et al. Kaposi's sarcoma: etiology and pathogenesis, inducing factors, causal associations, and treatments: facts and controversies. Clin Dermatol 2013;31:413-22. [Crossref] [PubMed]
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol 2013;68:313-31. [Crossref] [PubMed]
- Armstrong AW, Lam KH, Chase EP. Epidemiology of classic and AIDS-related Kaposi's sarcoma in the USA: incidence, survival, and geographical distribution from 1975 to 2005. Epidemiol Infect 2013;141:200-6. [Crossref] [PubMed]
- Mbulaiteye SM, Engels EA. Kaposi's sarcoma risk among transplant recipients in the United States (1993-2003). Int J Cancer 2006;119:2685-91. [Crossref] [PubMed]
- Yildiz F, Genc M, Akyurek S, et al. Radiotherapy in the management of Kaposi's sarcoma: comparison of 8 Gy versus 6 Gy. J Natl Med Assoc 2006;98:1136-9. [PubMed]
- Caccialanza M, Marca S, Piccinno R, et al. Radiotherapy of classic and human immunodeficiency virus-related Kaposi's sarcoma: results in 1482 lesions. J Eur Acad Dermatol Venereol 2008;22:297-302. [Crossref] [PubMed]
- Hamilton CR, Cummings BJ, Harwood AR. Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 1986;12:1931-5. [Crossref] [PubMed]
- Weshler Z, Loewinger E, Loewenthal E, et al. Megavoltage radiotherapy using water bolus in the treatment of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 1986;12:2029-32. [Crossref] [PubMed]
- Singh NB, Lakier RH, Donde B. Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiother Oncol 2008;88:211-6. [Crossref] [PubMed]


