
Symptom clusters in palliative-stage cancer correlate with proinflammatory cytokine cluster
Highlight box
Key findings
• A proinflammatory symptom cluster was found to correlate to a physical functioning and a gastro-intestinal-fatigue symptom cluster in patients with cancer in palliative stage. These findings suggest a connection between levels of pro-inflammatory cytokines in the blood of palliative cancer patients and symptom burden.
What is known and what is new?
• It is well established that cytokines cause different symptoms, it is also known that symptoms appear in clusters in several conditions. Different symptom clusters have been reported although a good definition still remains to be established. We identified three symptom clusters and four cytokine clusters. Correlations were found between the proinflammatory cytokine cluster and the physical functioning symptom cluster, and the gastro-intestinal-fatigue symptom cluster.
• We show for the first time that a cluster of cytokines correlate with clusters of symptoms in cancer.
• Knowing the causative role of cytokines opens new ways of treating adverse symptoms in various diseases, however verification in larger cohorts are needed.
Introduction
Patients with palliative-stage cancer are burdened by various manifestations both due to the cancer itself and other bodily processes related to the malignancy. As well as the local influence of the tumor or metastases, cancer patients report several general debilitating symptoms, due to the disease itself or from the treatments, with severe impact on quality of life (QOL). Associated symptoms common for all types of cancer are fatigue, pain, insomnia, gastrointestinal symptoms, anxiety and depressed mood (1). In advanced cancer, a patient may experience more than 13 symptoms simultaneously (2).
The symptoms reported by cancer patients share many features with the sickness behavior consisting of a coordinated set of adaptive behavioral changes that develop in people during an infection. This includes lethargy, depression, anxiety, malaise, loss of appetite, sleepiness, hyperalgesia, reduced hygiene and failure to concentrate. It is well established that this sickness behavior is a result of cytokines acting on the brain aiming to optimize the recovery of the individual (3). In end stage cancer, however, these symptoms seem to have no benefit for the patient—they contribute instead to severe suffering.
Many of these cancer-associated symptoms result from the inflammatory reaction given by a tumor invading a host. Increasing knowledge about how inflammation affects the progress of cancer has led to inflammation being described as the disease’s seventh hallmark (4). The inflammatory reaction is driven by cytokines and chemokines produced by the host, while the tumor itself also hijacks the system and produces cytokines to suppress the host’s antitumor immunity and mediate migration, invasion and metastasis of malignant cells (5). Although different tumors have their own characteristics, several common features may be identified, and with increased knowledge of the connection between cytokines and symptoms, we may find new ways to increase the QOL in cancer patients. In fact, several available anti-cytokine treatments used in other contexts have been proven to reduce various symptoms. For instance, anti-tumor necrosis factor (TNF)-α and interleukin (IL)-6 inhibitors both improved depressive symptoms, and anti-TNF-α treatment reduced the fatigue experienced by cancer patients (6,7).
Generally speaking, patients experience several symptoms, and it would be beneficial to find strategies to treat them concurrently. Multiple concurrent symptoms are thought to function as symptom clusters. A cluster can contain two or more related symptoms which may or may not suggest a common etiology or underlying mechanism (8). There is presently no consensus on the definition of specific clusters (9). However, symptoms such as anxiety-fatigue-breathlessness, nausea-vomiting-appetite loss, and pain-fatigue-sleep disturbance are often reported as clusters in several studies (10-12). The result may vary depending on the method used, and more studies are needed to define clusters and find methods of treatment aimed at relieving patients’ symptoms as much as possible.
Individual cytokines have been shown to correlate with specific symptoms (13). For example, TNF was shown to correlate with depression, and interleukin-1 receptor antagonist (IL-1RA) with fatigue. IL-6 is thought to predict worsening of symptoms but also more specifically affect appetite (14-17). Cluster analysis of cytokine levels in patients with chronic lymphocytic leukemia or breast cancer revealed clusters that were associated with prognosis and overall survival (18-20). To the best of our knowledge, no studies have reported correlation of cytokine clusters with symptom clusters. However, clusters of cytokine gene expression have been compared to symptoms. For instance, in breast cancer patients, the genetic variations in IL6, IL-13, and TNF-α were associated with the predefined symptom cluster of pain, fatigue, sleep disturbance, and depression (21).
This study aimed to identify symptom clusters and cytokine clusters in Swedish patients with palliative-stage cancer. Furthermore, we investigated correlations between the identified symptom clusters and cytokine clusters. Data and samples were collected at two time points in order to explore changes over time. PCA was carried out to identify symptom and cytokine clusters, followed by an exploration of associations between identified symptom and cytokine clusters. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-974/rc).
Methods
Study population and procedures
Patients were recruited consecutively from three palliative home care units and one department of internal medicine located in southeast Sweden between April 2016 and April 2018: Department of Geriatrics and Palliative Medicine, The County Hospital in Kalmar; Department of Advanced Home Care, Linköping University Hospital; Department of Advanced Home Care and Department of Internal Medicine in Vrinnevi Hospital, Norrköping. An overview of study enrollment is presented in Table 1.
Table 1
| Included | Excluded | % | |
|---|---|---|---|
| Screened | 493 | ||
| Not included according to criteria | 33 | ||
| Remaining included | 460 | ||
| Included | |||
| Excluded according to criteria | 219 | 48% of included | |
| Remaining included | 241 | 52% of included | |
| PP | |||
| Declined | 90 | 37% of PP | |
| Deceased before asked to participate | 7 | 3% of PP | |
| Accepted but did not participate | 34 | 14% of PP | |
| Participated in the study | 110 | 46% of PP |
PP, possible participants.
Data collection
A research nurse was recruited from each medical unit. The nurses screened inpatients consecutively according to inclusion and exclusion criteria—see Table 2. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients prior to their entering the study. The study was approved by the Regional Ethical Review Board in Linköping (application No. 2015/383-31). Each patient was assigned an ID-number, and all analysis of data was performed blinded. Medical data (e.g., type of malignancy, metastasis, corticosteroid treatment, nonsteroidal anti-inflammatory drugs (NSAID) treatment, antibiotics, specific cancer treatment) were collected from the medical record by the nurse, and demographic data (e.g., home situation, educational background and smoking) were obtained from the patient. The nurse visited the patients in their homes on two occasions, with four weeks between the visits. On each visit, the patient answered the EORTC Quality of Life Questionnaire Core 15 Palliative Care (EORTC QLQ-C15-PAL), and body temperature was measured in the right ear. Blood samples were taken at the end of the visit. Analysis of C-reactive protein (CRP), lactate dehydrogenase (LD), albumin and procalcitonin was performed on serum using accredited routine methods at two clinical laboratories.
Table 2
| Inclusion criteria | Exclusion criteria |
|---|---|
| At least 18 years old | Hematologic malignancy |
| Palliative-stage cancer | Pronounced leukopenia (LPK ≤1.0×109/L) |
| Antibiotic treatment for ongoing infection | |
| Cognitive impairment* | |
| Patient too weak for the study* | |
| Patient’s Swedish insufficient* |
*, according to the patient’s nurse or physician. LPK, leukocyte count.
EORTC QLQ-C15-PAL questionnaire
The EORTC-QLQ-C15-PAL consists of 15 questions (Q1–15) that refer to 2 functional scales (physical functioning Q1–3 and emotional functioning Q13, 14), 2 symptom scales (pain Q5, 12 and fatigue Q7, 11), 5 single-symptom items (shortness of breath Q4, trouble sleeping Q6, loss of appetite Q8, nausea Q9, and constipation Q10), and the global QoL scale Q15. Fourteen items are rated from 1 (not at all) to 4 (very much), and QOL is rated from 1 (very poor) to 7 (excellent) (22). Question number 15 addressing QOL was excluded from the cluster analysis, since it has a different scaling.
Cytokine measurement
Blood samples for cytokine measurements were collected in ethylenediaminetetraacetic acid (EDTA) tubes. The tubes were stored on ice until processing in the laboratory. Within four hours of sampling, the blood samples were centrifuged (1,800 G, 10 minutes at 4 ℃) and frozen at −70 ℃. Until analysis, all samples were stored at the Linköping Biobank Facility.
Three patients were excluded from cytokine measurements due to hepatitis infection (two patients both at baseline and four weeks, one patient only baseline). Cytokines were analyzed using MSD technology (Mesoscale, Rockville, MD, USA). The following human kits were used: R-PLEX TNF-RII Antibody Set, U-PLEX Biomarker Group 1 Assays {interferon (IFN)-α2a IL-1RA, IL-3, IL-7, IL-9, IL-16, IL-17A, IL-17F, IFN-γ induced protein 10 (IP-10), V-PLEX Proinflammatory panel 1 (IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α), and U-PLEX TGF-β Combo [transforming growth factor (TGF)-β1, β2, and β3]}. The cytokines were selected based on previous research on cancer related inflammation and symptoms (16,17,23). The cytokines IL-4, TGF-β1 and TGF-β2 were subsequently excluded from further analysis due to low readings, resulting in measurements from 20 cytokines used for analysis. Cytokine results from one patient were excluded due to outlying values.
Statistical analysis
Paired samples t-tests with Bonferroni-correction were performed for each question and the cytokine concentrations were analyzed using Wilcoxon signed-rank tests with Bonferroni-correction, investigating differences between baseline and the four weeks time point. Non-parametric tests were chosen for the cytokine measurements because the data was not normally distributed.
Cytokine values were normalized by calculating z-scores with the formula: , and PCA of the cytokine data was performed using the z-scores. SPSS software was used for all statistical analysis.
PCA
Baseline
Questionnaire data of question 1–14 EORTC QLQ-C15-PAL were subjected to factor analysis using PCA and orthogonal Varimax rotation. The Kaiser-Meyer-Olkin measure (KMO) was 0.754, indicating that the data were sufficient for factor analysis. 100% of KMO values for the individual items were above 0.5. The Bartlett’s test of sphericity χ2(91)=507.872, P=0.000 showed that there were patterned relationships between the items. Iterating cut-off and numbers of factors resulted in three factors with at least three unique components that explain a cumulative variance of 53.6%. The scree plot confirmed the findings of retaining three factors.
PCA of cytokine values with orthogonal Varimax rotation gave a KMO of 0.696. Ninety percent of KMO values for the individual items were above 0.5 (IL-17F and TNF-RII, which did not fit into the extracted components displayed values below 0.5). The Bartlett’s test of sphericity χ2(190)=1,488.010, P=0.000 showed that there were patterned relationships between the items. Iterating cut-off and numbers of factors resulted in three factors with at least five unique components that explain a cumulative variance of 55.4%. The scree plot confirmed the findings of retaining three factors.
Four weeks
The same analysis was performed on data from the four-week time point. Analyzing the questionnaire, KMO was 0.785. Ninety-five percent of KMO values for the individual items were above 0.5. The Bartlett’s test of sphericity: χ2(91)=478.450, P=0.000. Iterating cut-off and numbers of factors resulted in three factors with at least three unique components that explain a cumulative variance of 57.7%. The scree plot confirmed the findings of retaining three factors.
PCA on cytokine values with orthogonal Varimax rotation resulted in four factors with at least two unique components using a cut-off that explains a cumulative variance of 70.2%. KMO was 0.819, and 100% of KMO values for the individual items were above 0.5. The Bartlett’s test of sphericity: χ2(190)=1,753.392, P=0.000. The scree plot confirmed the findings of retaining four factors.
Correlation of clusters
The data obtained from the PCA were used for correlation analysis of the different clusters. Linear correlation was performed calculating the Pearson correlation coefficient, r. Thus, clusters 1–3 from the symptom clusters at baseline were correlated with clusters 1–3 from the cytokine clusters at baseline, and consequently clusters 1–3 of symptoms and 1–4 of cytokines at four weeks were correlated.
Results
There were 110 patients that completed EORTC QLQ-C15-PAL at baseline. The average age was 68 years, with equal distribution between sexes (49% male, 51% female). Several different primary tumors were represented, and 85.5% of the patients presented with metastases. Of the 110 patients, 90 patients (82%) participated four weeks later. The patient characteristics are summarized in Table 3. The drop in participation at the second time point was, in all cases, due to patients having deceased. EORTC QLQ-C15-PAL scores are summarized in Table 4. The symptoms with highest score at both time points were tiredness, fatigue and feebleness. The majority of scores, such as shortness of breath, trouble taking short walks, tenseness and fatigue, were higher (i.e., had worsened) after four weeks, whereas constipation was improved, although no statistical significance was seen using paired t-test with Bonferroni correction (see Table 4).
Table 3
| Characteristics | Baseline | 4 weeks |
|---|---|---|
| Total patients | 110 | 90 |
| Age (years) | ||
| Mean ± SD | 68±12.3 | 69±11.9 |
| Median [range] | 70 [23–89] | 71 [23–88] |
| Gender | ||
| Male | 54 (49%) | 45 (50%) |
| Female | 56 (51%) | 45 (50%) |
| Primary cancer site | ||
| Lung | 16 | |
| Prostate | 12 | |
| Large intestine | 12 | |
| Breast | 10 | |
| Skin | 7 | |
| Ventricle/esophagus | 7 | |
| Peritoneum | 5 | |
| Pancreas | 5 | |
| Kidney | 4 | |
| Gallbladder/duct | 4 | |
| Liver | 4 | |
| Brain | 4 | |
| Bone | 4 | |
| Other | 16 | |
| Patients with metastases | 94 | 75 |
| Site of metastases | ||
| Liver | 34 | 26 |
| Lung | 23 | 19 |
| Skeletal | 38 | 32 |
| Abdomen | 14 | 11 |
| Lymph node | 20 | 17 |
| Brain | 12 | 9 |
| Adrenal gland | 5 | 3 |
| Skin | 4 | 3 |
| Other/unknown | 10 | 9 |
| None | 16 | 15 |
Table 4
| Question | Mean ± SD | Paired t-test, total (n) | ||||
|---|---|---|---|---|---|---|
| Baseline | 4 weeks | P | Baseline | 4 weeks | ||
| Q1 short walk | 2.15±1.15 | 2.47±1.30 | 0.007 | 109 | 90 | |
| Q2 in bed | 2.55±0.93 | 2.72±0.91 | 0.050 | 109 | 90 | |
| Q3 need help | 1.52±0.91 | 1.64±0.99 | 0.064 | 110 | 90 | |
| Q4 shortness of breath | 1.86±0.94 | 2.14±1.10 | 0.005 | 110 | 90 | |
| Q5 pain | 2.15±0.98 | 2.15±0.94 | 0.913 | 110 | 89 | |
| Q6 trouble sleeping | 1.72±0.94 | 1.61±0.83 | 0.300 | 110 | 90 | |
| Q7 feebleness | 2.52±0.87 | 2.57±0.94 | 0.581 | 108 | 90 | |
| Q8 lacking appetite | 1.83±1.00 | 1.91±1.07 | 0.599 | 110 | 90 | |
| Q9 nausea | 1.57±0.71 | 1.62±0.78 | 0.518 | 110 | 90 | |
| Q10 constipation | 1.68±0.95 | 1.40±0.80 | 0.009 | 110 | 90 | |
| Q11 tiredness | 2.69±0.77 | 2.85±0.97 | 0.193 | 109 | 90 | |
| Q12 pain interference | 1.75±1.02 | 1.86±1.05 | 0.229 | 110 | 90 | |
| Q13 tenseness | 1.57±0.77 | 1.80±0.99 | 0.013 | 110 | 90 | |
| Q14 depressed mood | 1.84±0.86 | 1.92±0.93 | 0.572 | 110 | 90 | |
| Q15 total QOL | 4.08±1.24 | 4.06±1.36 | 0.831 | 110 | 90 | |
The P value for each pair is reported, significance level is P<0.003 according to Bonferroni correction analyzing 15 parameters. QOL, quality of life; SD, standard deviation.
The data from the symptom questionnaire were subjected to factor reduction using PCA. Three clusters were identified at both timepoints (Tables 5-7). At baseline Cluster 1 included symptoms of shortness of breath (Q4), pain (Q5), trouble sleeping (Q6), feebleness (Q7) and pain interfering with daily activities (Q12). Cluster 2 was represented by lack of appetite (Q8), nausea (Q9), tiredness (Q11) and depressed mood (Q14), whereas Cluster 3 described trouble taking a short walk (Q1), the patient needing to stay in bed during the day (Q2) and needing help with basic daily activities (Q3). Questions 1–3 in Cluster 3 are defined as “Physical functioning” according to EORTC C15-PAL (22).
Table 5
| Cluster | Items | Component 1 | Component 2 | Component 3 | Final communality |
|---|---|---|---|---|---|
| 1 | Q4 shortness of breath | 0.525 | −0.061 | 0.065 | 0.284 |
| Q5 pain | 0.622 | 0.414 | 0.176 | 0.589 | |
| Q6 trouble sleeping | 0.660 | −0.046 | −0.213 | 0.483 | |
| Q7 feebleness | 0.631 | 0.309 | 0.413 | 0.664 | |
| Q12 pain interference | 0.676 | 0.426 | 0.155 | 0.663 | |
| 2 | Q8 lacking appetite | −0.162 | 0.755 | 0.161 | 0.622 |
| Q9 nausea | 0.048 | 0.660 | −0.390 | 0.590 | |
| Q11 tiredness | 0.430 | 0.588 | 0.184 | 0.564 | |
| Q14 depressed mood | 0.294 | 0.561 | 0.232 | 0.455 | |
| 3 | Q1 short walk | 0.514 | −0.081 | 0.611 | 0.643 |
| Q2 in bed | 0.515 | 0.282 | 0.572 | 0.671 | |
| Q3 need help | −0.005 | 0.142 | 0.823 | 0.697 | |
| Q10 constipation | −0.119 | 0.382 | 0.348 | 0.282 | |
| Q13 tenseness | 0.297 | 0.443 | 0.083 | 0.291 |
Table 6
| Cluster | Items | Component 1 | Component 2 | Component 3 | Final communality |
|---|---|---|---|---|---|
| 1 | Q5 pain | 0.712 | −0.164 | 0.166 | 0.561 |
| Q6 trouble sleeping | 0.549 | 0.051 | 0.006 | 0.304 | |
| Q12 pain interference | 0.693 | 0.372 | 0.139 | 0.638 | |
| Q13 tenseness | 0.743 | −0.142 | 0.173 | 0.603 | |
| Q14 depressed mood | 0.709 | 0.142 | 0.284 | 0.604 | |
| 2 | Q4 shortness of breath | −0.086 | 0.785 | −0.051 | 0.627 |
| Q7 feebleness | 0.304 | 0.637 | 0.264 | 0.568 | |
| Q8 lacking appetite | 0.400 | 0.537 | 0.072 | 0.454 | |
| Q11 tiredness | 0.273 | 0.697 | 0.313 | 0.658 | |
| 3 | Q1 short walk | 0.102 | 0.532 | 0.682 | 0.759 |
| Q2 in bed | 0.246 | 0.432 | 0.704 | 0.743 | |
| Q3 need help | 0.021 | 0.109 | 0.806 | 0.663 | |
| – | Q9 nausea | 0.530 | 0.307 | −0.278 | 0.452 |
| – | Q10 constipation | 0.217 | −0.356 | 0.517 | 0.441 |
Table 7
| Quest | Cluster | ||
|---|---|---|---|
| 1st cluster | 2nd cluster | 3rd cluster | |
| Baseline | Q4, Q5, Q6, Q7, Q12 | Q8, Q9, Q11, Q14 | Q1, Q2, Q3 |
| 4 weeks | Q5, Q6, Q12, Q13, Q14 | Q4, Q7, Q8, Q11 | Q1, Q2, Q3 |
At four weeks, Cluster 1 included the two pain questions (Q5 and 12) as well as trouble sleeping (Q6) with the addition of tenseness (Q13) and depressed mood (Q14). Cluster 2 contained Q8 lacked appetite and Q11 being tired as baseline, with the addition of Q4 shortness of breath and Q7 feebleness. Cluster 3 at four weeks was the same as for baseline. The clusters are summarized in Table 7.
Blood samples for cytokine measurements were drawn on the same visit as the questionnaire was conducted. Cytokine data is summarized in Table 8 and Figure S1. There were no significant changes between measurements at baseline and at 4 weeks. The cytokine data were subjected to factor reduction by PCA in a similar manner as the symptom data. Three cytokine clusters were identified at baseline and 4 cytokine clusters at 4 weeks, see Tables 9,10 for details.
Table 8
| Cytokine | Mean ± SD (pg/mL) | Median (pg/mL) | Wilcoxon | Range (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | Baseline | 4 weeks | P | Baseline | 4 weeks | ||||
| IFN-γ | 8.56±7.35 | 10.88±16.65 | 5.70 | 6.11 | 0.79 | 0.04–37.36 | 1.67–114.38 | |||
| IL-10 | 1.59±1.80 | 1.30±1.38 | 0.92 | 0.91 | 0.62 | 0.21–13.6 | 0.31–10.07 | |||
| IL-12p70 | 0.73±0.81 | 0.74±0.88 | 0.48 | 0.43 | 0.39 | 0.03–4.95 | 0.12–5.18 | |||
| IL-13 | 4.51±4.63 | 4.82±4.77 | 2.32 | 2.33 | 0.77 | 0.04–27.36 | 0.48–20.8 | |||
| IL-1β | 0.31±0.34 | 0.26±0.26 | 0.18 | 0.17 | 0.05 | 0.00–1.56 | 0.02–1.56 | |||
| IL-2 | 0.75±1.25 | 0.68±1.10 | 0.34 | 0.32 | 0.93 | 0.03–7.37 | 0.02–6.93 | |||
| IL-6 | 5.23±8.42 | 3.90±3.69 | 2.66 | 2.66 | 0.35 | 0.27–56.06 | 0.31–19.31 | |||
| IL-8 | 10.78±22.87 | 11.09±23.05 | 4.29 | 4.15 | 0.02 | 0.76–173.68 | 0.72–179.23 | |||
| TNF-α | 3.02±1.80 | 3.00±1.59 | 2.52 | 2.68 | 0.36 | 0.00–12.11 | 0.70–9.11 | |||
| TGF-β3 | 2.43±2.76 | 2.32±2.02 | 1.50 | 1.59 | 0.16 | 0.06–14.98 | 0.09–9.04 | |||
| IFN-α2a | 6.58±7.35 | 6.08±5.47 | 4.59 | 4.62 | 1.00 | 0.37–39.20 | 0.16–32.14 | |||
| IL-16 | 228.27±93.31 | 227.39±100.42 | 214.67 | 211.96 | 0.42 | 68.78–559.63 | 62.82–517.08 | |||
| IL-17A | 49.00±32.53 | 45.01±27.86 | 42.46 | 39.55 | 0.57 | 9.69–184.85 | 7.76–150.22 | |||
| IL-1RA | 687.81±1,029.15 | 478.22±399.44 | 373.89 | 323.71 | 0.72 | 135.22–6,526.68 | 112.69–399.44 | |||
| IL-3 | 85.09±99.09 | 77.04±74.92 | 47.94 | 46.93 | 0.96 | 5.76–510.88 | 0.72–45.41 | |||
| IL-7 | 8.45±8.79 | 7.74±6.96 | 5.35 | 5.18 | 0.93 | 1.01–56.28 | 0.72–45.41 | |||
| IL-9 | 1.48±1.46 | 1.25±1.22 | 1.03 | 0.92 | 0.25 | 0.04–7.85 | 0.12–6.44 | |||
| IP-10 | 677.26±816.63 | 662.80±890.80 | 482.40 | 396.52 | 0.70 | 55.46–6,553.18 | 92.25–5,625.3 | |||
| IL-17F | 60.78±66.79 | 44.77±52.52 | 46.11 | 47.14 | 0.40 | 0.85–345.37 | 0.78–205.16 | |||
| TNF-RII | 19,875.4±14,596.2 | 19,216.0±14,196.2 | 17,245.5 | 16,754.1 | 0.89 | 26.38–77,340.6 | 157.36–62,232 | |||
Significance, P, referring to significance using Wilcoxon signed-rank test. Bonferroni corrected P value based on 20 cytokines gives significant difference if P<0.0025. SD, standard deviation; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; TGF, transforming growth factor; IP, induced protein.
Table 9
| Cluster | Items | Component 1 | Component 2 | Component 3 | Final communality |
|---|---|---|---|---|---|
| 1 | IFN-α2a | 0.959 | 0.070 | 0.107 | 0.935 |
| 1 | IL-3 | 0.956 | 0.015 | 0.055 | 0.916 |
| 1 | IL-7 | 0.915 | −0.010 | 0.139 | 0.856 |
| 1 | TGF-β3 | 0.808 | −0.019 | 0.038 | 0.655 |
| 1 | IL-17A | 0.722 | 0.244 | 0.366 | 0.714 |
| 1 | IL-9 | 0.701 | −0.036 | 0.220 | 0.541 |
| 2 | IL-12p70 | −0.054 | 0.877 | −0.070 | 0.777 |
| 2 | IL-2 | −0.047 | 0.821 | −0.003 | 0.677 |
| 2 | IL-13 | −0.049 | 0.722 | −0.068 | 0.528 |
| 2 | IFN-γ | 0.041 | 0.711 | 0.311 | 0.604 |
| 2,3 | IL-1β | 0.094 | 0.621 | 0.513 | 0.657 |
| 2 | IL-10 | 0.045 | 0.476 | 0.250 | 0.292 |
| 3 | TNF-α | −0.007 | 0.408 | 0.772 | 0.762 |
| 3 | IL-16 | 0.194 | 0.062 | 0.717 | 0.555 |
| 3 | IL-8 | 0.017 | −0.083 | 0.647 | 0.426 |
| 3 | IP-10 | 0.147 | 0.055 | 0.589 | 0.371 |
| 3 | IL-1RA | 0.136 | −0.075 | 0.551 | 0.328 |
| 3 | IL-6 | 0.025 | 0.240 | 0.548 | 0.358 |
| – | TNF-RII | 0.124 | −0.074 | 0.258 | 0.087 |
| – | IL-17F | 0.064 | 0.180 | −0.061 | 0.040 |
IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor; IP, induced protein.
Table 10
| Cluster | Items | Component 1 | Component 2 | Component 3 | Component 4 | Final communality |
|---|---|---|---|---|---|---|
| 1 | IL-3 | 0.960 | 0.045 | 0.045 | 0.074 | 0.932 |
| 1 | IL-7 | 0.938 | 0.165 | 0.054 | 0.015 | 0.910 |
| 1 | IFN-α2a | 0.935 | 0.115 | 0.042 | 0.114 | 0.903 |
| 1 | IL-9 | 0.850 | 0.171 | 0.049 | 0.227 | 0.806 |
| 1 | TGF-β3 | 0.816 | 0.127 | 0.128 | −0.102 | 0.709 |
| 1 | IL-17A | 0.687 | 0.466 | 0.008 | 0.242 | 0.747 |
| 2 | IL-8 | 0.120 | 0.803 | −0.070 | −0.064 | 0.667 |
| 2 | IL-6 | 0.111 | 0.734 | 0.140 | 0.035 | 0.572 |
| 2 | IL-1RA | 0.151 | 0.719 | 0.078 | 0.121 | 0.560 |
| 2 | TNF-α | 0.201 | 0.697 | 0.444 | 0.355 | 0.849 |
| 2 | IL-10 | 0.081 | 0.625 | 0.339 | 0.058 | 0.515 |
| 2 | TNF-RII | 0.138 | 0.548 | 0.262 | 0.240 | 0.446 |
| 2 | IL-16 | 0.421 | 0.488 | 0.253 | 0.308 | 0.574 |
| 3 | IL-12p70 | 0.008 | 0.088 | 0.935 | 0.033 | 0.883 |
| 3 | IL-2 | −0.013 | 0.096 | 0.854 | 0.017 | 0.739 |
| 3 | IL-13 | 0.021 | 0.138 | 0.797 | 0.171 | 0.684 |
| 3 | IL-1β | 0.130 | 0.472 | 0.695 | −0.049 | 0.725 |
| 4 | IFN-γ | 0.088 | 0.008 | 0.238 | 0.864 | 0.811 |
| 4 | IP-10 | 0.147 | 0.299 | 0.016 | 0.844 | 0.823 |
| – | IL-17F | 0.111 | 0.080 | 0.382 | 0.112 | 0.177 |
IL, interleukin; IFN, interferon; TGF, transforming growth factor; TNF, tumor necrosis factor; IP, induced protein.
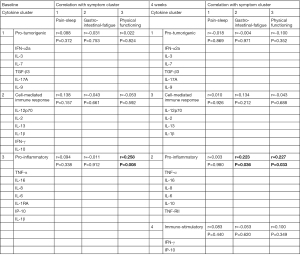
The clusters obtained from the symptom analysis were correlated to the clusters obtained from the cytokine data. At baseline, a significant correlation was obtained between Symptom Cluster 3 (Physical functioning) and Cytokine Cluster 3 (r=0.258, P=0.008). At four weeks, the Physical functioning symptom cluster correlated with Cytokine Cluster 2 (r=0.227, P=0.033). There was also a significant correlation between Cytokine Cluster 2 and Symptom Cluster 2 (r=0.223, P=0.036), summarized in Figure 1.
Discussion
Three symptom clusters were extracted at both baseline and the four-week timepoint. The first cluster at both time points included symptoms of pain and sleep disturbance. The second cluster included gastro-intestinal and fatigue symptoms, and the third cluster at both time points represented physical functioning symptoms. Three cytokine clusters were identified at both time points, representing pro-tumorigenic, cell-mediated immune responses and proinflammatory cytokines. Additionally, at the four-week timepoint, a fourth cluster with immunostimulatory cytokines was identified. The proinflammatory cytokine cluster correlated with the physical functioning and gastro-intestinal and fatigue symptom cluster.
Several studies have investigated symptom clusters in patients with cancer, but no overall accepted definition of the clusters has been presented. However, some clusters are reported in the literature more consistently than others, such as a gastro-intestinal symptom cluster with nausea, vomiting and occasional lack of appetite; a psychoneurological cluster including pain, fatigue and sleep disturbance sometimes associated with mood disturbances; and a respiratory symptom cluster with breathlessness, fatigue, anxiety and coughing (11). One problem with comparing different studies is that different methods have been used to evaluate the symptoms. This makes direct comparison difficult. In the present study the EORTC QLQ-C15-PAL—an internationally validated palliative care-specific health-related QOL instrument (22)—was used. This is a test with a small amount of questions (15), which is important since the patients are often exhausted and have limited amounts of energy for answering questionnaires.
The present study identified three symptom clusters (I) pain-sleep disorder, (II) gastro-intestinal-fatigue, (III) physical functioning. Comparing the results of this study with one by Ganesh et al. which also used EORTC QLQ-C15-PAL at three different time-points in patients undergoing palliative radiotherapy reveals several interesting common features (12). As in the present study, the questionnaire data were analyzed by PCA, and several symptoms can be identified with similar distribution. The physical functioning symptom cluster isolated in the present study (questions 1–3) was also identified in the Ganesh study. In addition, questions 5, 6 and 12 addressing pain and trouble sleeping, as well as questions 4 and 7 representing shortness of breath and feebleness, gathered in the same clusters respectively in both studies. Gastrointestinal symptoms (questions 8 and 9) were associated in the Ganesh study, whereas the present study only demonstrated this at the baseline timepoint (22).
The present study identified three cytokine clusters at baseline and four at four weeks. Cluster 1 at both timepoints contained the same cytokines: IFN-α2a, IL-3, IL-7, TGF-β 3, IL-17A and IL-9. Although the cytokine signaling processes are complex and often pleiotropic, there are studies suggesting that pro-tumorigenic activities may be represented in this cluster. TGF-β plays a key role in tumor-induced immunosuppression and stimulates production of factors that promote tumor growth—it is thought to be involved in tumor angiogenesis together with IL-3 (24). In addition, IL-17, recovered in the same cluster, induces VEGF which can induce TGF-β mediating angiogenesis (25,26). TGF-β levels in serum correlate with poor prognosis and increase the tumor’s metastatic potential (27).
IL-12p70, IL-2 and IFN-γ cytokines found in cluster 2 at baseline are cytokines with important roles in cell-mediated immune response against tumor cells (17). These cytokines have been shown to inhibit tumor growth and have been used experimentally as immunotherapy against cancer (28,29). In clinical tests, IL-2 administration resulted in reduced cognitive function and depressive symptoms (30,31).
The third cluster at baseline contains the classic pro-inflammatory cytokines IL-1β, IL-6 and TNF-α. IL-16 in the same cluster can stimulate secretion of these proinflammatory cytokines from monocytes and maturing macrophages (32). IL-8 is also considered to be a proinflammatory cytokine that increases in parallel with IL-6 and TNF-α as the cancer progresses (33,34). Pro-inflammatory cytokines are important for tumor growth, angiogenesis, and spreading of the tumor by metastases (35).
At the four-week time point, a fourth cluster was isolated containing IFN-γ and IP-10. IP-10, also known as CXCL10, is secreted in response to IFN-γ (36), known for its immunostimulatory properties, may also have tumor-promoting properties helping in the selection of tumor cells with immuno-evasive phenotype (37).
A significant correlation was observed between the proinflammatory cytokine cluster (cytokine cluster 3) and the physical functioning symptom cluster as well as the second symptom cluster (including fatigue questions) at the four-week time point. These findings indicate a connection between proinflammatory activation and diminished everyday functioning. These findings are in line with the sickness-behavior initiated by the proinflammatory cytokines, resulting in weakness, inability to concentrate, decreased interest in one’s surroundings and anhedonia (38). Inhibiting the circulating cytokines may thus improve symptoms in patients with advanced cancer. Several anti-cytokine treatments are used for specific inflammatory conditions and the side-effects give clues as to how specific cytokines may impact symptom burden. For instance, fatigue has been linked to TNF-α. Patients with docetaxel treatment that received anti-TNF-α treatment using etanercept experienced less fatigue, while patients with rheumatoid arthritis—a disease where fatigue is a major burden for the patients—reported significantly less fatigue after anti-TNF treatment (7,39,40). IL-6 can also cause fatigue, as demonstrated in patients with Castleman disease, a lymphoproliferative disorder characterized by increased IL-6 production, where IL-6 receptor administration reduced fatigue reported by the patients. Additionally, IL-6 has been shown to affect appetite and correlate with depression in cancer patients (39,41).
Few studies have investigated the association of symptom clusters with cytokine levels in the blood. In a Chinese study comparing symptom clusters with cytokine levels in 170 cancer patients, IL-6 was found to be associated with reported physical performance status, whereas no association was reported for TNF-α or IL-1β in this study (42). One study investigated the association between cytokine genes and symptom clusters, reporting an association between IL-6, TNF-α and IL-1β genes and the symptom cluster of pain, fatigue, sleep disturbance and, depression—however, the translation of the gene-expression to circulating plasma cytokines makes it difficult to compare data (21).
This study has shown correlations between cytokine clusters and symptom clusters, which may indicate that anti-cytokine treatment may have a place in palliative care of cancer patients.
It must, however, be remembered that other interventions may have good effect on the symptom burden. For instance, methylphenidate can improve fatigue and depression (43,44). Non-pharmacological treatments such as customized exercise, massage, psycho-educational and behavioral interventions have also proven effective (45-50). An optimal treatment should be individualized using a combination of the different options available.
The study population consisted of 110 cancer patients in a palliative disease stage. Although the heterogeneity, with many different cancer types, may have lowered the power to detect symptom and cytokine clusters related to specific kinds of cancer, we believe the results may be broadly generalizable for both symptom clusters and cytokine clusters, indicating common features in general palliative cancer care populations.
Conclusions
In conclusion, a pro-inflammatory symptom cluster was found to correlate with physical functioning symptoms as well as a fatigue symptom cluster. These findings suggest a connection between levels of pro-inflammatory cytokines in the blood of palliative cancer patients and symptom burden. One way to treat those symptoms may be to reduce circulating cytokine levels in the blood.
Acknowledgments
The abstract has been published in Mendeley Data (https://data.mendeley.com/datasets/p8g3znzspb), under a Creative Commons Attribution 4.0 International license (CC BY 4.0)
Funding: Financial support was received from the Medical Research Council of Southeast Sweden (FORSS, grant Nos. FORSS-562671, FORSS-659831, FORSS-757591, and FORSS-931943), ALF Grants (Nos. LIO-606981, LIO-696011), and Research and scholarship management in Region Östergötland (grant Nos. LIO-647161, LIO-759641).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-974/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-974/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-974/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-974/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients prior to their entering the study. The study was approved by the Regional Ethical Review Board in Linköping (application No. 2015/383-31).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 2014;106:dju129. [Crossref] [PubMed]
- Chang VT, Hwang SS, Feuerman M, et al. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer 2000;88:1175-83. [Crossref] [PubMed]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 2007;21:153-60. [Crossref] [PubMed]
- Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073-81. [Crossref] [PubMed]
- Lan T, Chen L, Wei X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021;10:100. [Crossref] [PubMed]
- Kappelmann N, Lewis G, Dantzer R, et al. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 2018;23:335-43. [Crossref] [PubMed]
- Monk JP, Phillips G, Waite R, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol 2006;24:1852-9. [Crossref] [PubMed]
- Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs 2001;33:668-76. [Crossref] [PubMed]
- Barsevick A. Defining the Symptom Cluster: How Far Have We Come? Semin Oncol Nurs 2016;32:334-50. [Crossref] [PubMed]
- Dong ST, Butow PN, Costa DS, et al. Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage 2014;48:411-50. [Crossref] [PubMed]
- Kwekkeboom KL. Cancer Symptom Cluster Management. Semin Oncol Nurs 2016;32:373-82. [Crossref] [PubMed]
- Ganesh V, Zhang L, Wan BA, et al. Symptom clusters using the EORTC QLQ-C15-PAL in palliative radiotherapy. Ann Palliat Med 2018;7:192-204. [Crossref] [PubMed]
- Patton R, Paval DR, McDonald JJ, et al. Relationship between cytokines and symptoms in people with incurable cancer: A systematic review. Crit Rev Oncol Hematol 2021;159:103222. [Crossref] [PubMed]
- Lyon DE, Schubert C, Taylor AG. Pilot study of cranial stimulation for symptom management in breast cancer. Oncol Nurs Forum 2010;37:476-83. [Crossref] [PubMed]
- Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102-9. [Crossref] [PubMed]
- Paulsen Ø, Laird B, Aass N, et al. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS One 2017;12:e0177620. [Crossref] [PubMed]
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013;14:e218-28. [Crossref] [PubMed]
- Yan XJ, Dozmorov I, Li W, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood 2011;118:5201-10. [Crossref] [PubMed]
- Kawaguchi K, Sakurai M, Yamamoto Y, et al. Alteration of specific cytokine expression patterns in patients with breast cancer. Sci Rep 2019;9:2924. [Crossref] [PubMed]
- Jabeen S, Espinoza JA, Torland LA, et al. Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. Oncoimmunology 2018;8:e1537691. [Crossref] [PubMed]
- Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs 2015;17:237-47. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Aaronson NK, et al. EORTC QLQ-C15-PAL: the new standard in the assessment of health-related quality of life in advanced cancer? Palliat Med 2006;20:59-61. [Crossref] [PubMed]
- Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887-99. [Crossref] [PubMed]
- Prud'homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 2007;87:1077-91. [Crossref] [PubMed]
- Dentelli P, Rosso A, Calvi C, et al. IL-3 affects endothelial cell-mediated smooth muscle cell recruitment by increasing TGF beta activity: potential role in tumor vessel stabilization. Oncogene 2004;23:1681-92. [Crossref] [PubMed]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009;183:4169-75. [Crossref] [PubMed]
- Li J, Shen C, Wang X, et al. Prognostic value of TGF-beta in lung cancer: systematic review and meta-analysis. BMC Cancer 2019;19:691. [Crossref] [PubMed]
- Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med 2018;7:4509-16. [Crossref] [PubMed]
- Weiss JM, Subleski JJ, Wigginton JM, et al. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther 2007;7:1705-21. [Crossref] [PubMed]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med 2001;63:376-86. [Crossref] [PubMed]
- Capuron L, Ravaud A, Gualde N, et al. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology 2001;26:797-808. [Crossref] [PubMed]
- Mathy NL, Scheuer W, Lanzendörfer M, et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000;100:63-9. [Crossref] [PubMed]
- Ma Y, Ren Y, Dai ZJ, et al. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med 2017;26:421-6. [Crossref] [PubMed]
- Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 2017;60:24-31. [Crossref] [PubMed]
- Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [Crossref] [PubMed]
- Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer Oncol Lett 2011;2:583-9. (Review). [Crossref] [PubMed]
- Mojic M, Takeda K, Hayakawa Y. The Dark Side of IFN-gamma: Its Role in Promoting Cancer Immunoevasion. Int J Mol Sci 2017;19. [PubMed]
- Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum 2008;35:802-7. [Crossref] [PubMed]
- Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 2005;106:2627-32. [Crossref] [PubMed]
- Yount S, Sorensen MV, Cella D, et al. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol 2007;25:838-46. [PubMed]
- Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001;158:1252-7. [Crossref] [PubMed]
- Ji YB, Bo CL, Xue XJ, et al. Association of Inflammatory Cytokines With the Symptom Cluster of Pain, Fatigue, Depression, and Sleep Disturbance in Chinese Patients With Cancer. J Pain Symptom Manage 2017;54:843-52. [Crossref] [PubMed]
- Jiang Z, Butler-Bowen H, Rodriguez T, et al. Role of methylphenidate in the treatment of fatigue in advanced pancreatic cancer population. Ann Gastroenterol 2016;29:536-43. [Crossref] [PubMed]
- Lasheen W, Walsh D, Mahmoud F, et al. Methylphenidate side effects in advanced cancer: a retrospective analysis. Am J Hosp Palliat Care 2010;27:16-23. [Crossref] [PubMed]
- Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol 2004;22:507-16. [Crossref] [PubMed]
- Donovan HS, Ward SE, Sereika SM, et al. Web-based symptom management for women with recurrent ovarian cancer: a pilot randomized controlled trial of the WRITE Symptoms intervention. J Pain Symptom Manage 2014;47:218-30. [Crossref] [PubMed]
- McMillan SC, Small BJ. Using the COPE intervention for family caregivers to improve symptoms of hospice homecare patients: a clinical trial. Oncol Nurs Forum 2007;34:313-21. [Crossref] [PubMed]
- Knobf MT, Thompson AS, Fennie K, et al. The effect of a community-based exercise intervention on symptoms and quality of life. Cancer Nurs 2014;37:E43-50. [Crossref] [PubMed]
- Sturgeon M, Wetta-Hall R, Hart T, et al. Effects of therapeutic massage on the quality of life among patients with breast cancer during treatment. J Altern Complement Med 2009;15:373-80. [Crossref] [PubMed]
- Jarden M, Nelausen K, Hovgaard D, et al. The effect of a multimodal intervention on treatment-related symptoms in patients undergoing hematopoietic stem cell transplantation: a randomized controlled trial. J Pain Symptom Manage 2009;38:174-90. [Crossref] [PubMed]


