
Symptoms and disease severity in lung transplant candidates co-managed with palliative care
Highlight box
Key findings
• Symptom burden is high among instersitital lung disease (ILD) and chronic obstructive pulmonary disease (COPD) patients awaiting lung transplantation with the most common symptoms being dyspnea, cough, and fatigue.
• There was no association between the change in symptom burden and physiologic markers of disease severity (i.e., 6-minute walk distance, oxygen requirements, or respiratory exacerbations), in patients with COPD and ILD awaiting lung transplantation
What is known and what is new?
• It is known that lung transplant candidates have impaired quality of life and significant symptoms, often warranting palliative care support.
• We show that symptom burden correlates poorly with traditional markers of disease severity.
What is the implication, and what should change now?
• A tool such as the Edmonton Symptom Assessment Score can provide meaningful insight into the symptom burden of patients awaiting lung transplantation.
• Early palliative care referral of patients awaiting lung transplantation should be considered given the discordance between symptoms and disease severity.
Introduction
Interstitial lung disease (ILD) and chronic obstructive pulmonary disease (COPD) are lung diseases with global prevalence up to 71 per 100,000 people for ILD and 10,300 per 100,000 for COPD (1,2). Together, these two diseases represent the two most common indications for lung transplantation (3,4). Lung transplant (LTx) candidates experience significant physical and mental health sequelae, such as dyspnea, cough, anxiety, and depression (5-7). With respiratory disease progression, symptoms may progress and limit daily function, physical activity, and health related quality of life (HRQL) (8). Given the persistent symptom burden in LTx candidates, palliative care (PC) support is often undertaken to improve symptoms and daily function. PC is gaining increased recognition, with guidelines by the American College of Chest Physicians and American Thoracic Society, recommending that LTx candidates have access to palliative symptom-based therapies to target symptoms and improve physical function (9,10).
A common management strategy in PC has been the utilization of patient reported outcome measures to guide pharmacological and non-pharmacological strategies aimed at optimizing symptoms and emotional well-being. One such measure utilized in chronic lung disease is the Edmonton Symptom Assessment System (ESAS), which captures twelve domains, focused on respiratory symptoms, energy levels, gastrointestinal symptoms, pain and mental well-being (11). In COPD patients, the most common symptoms reported are fatigue, dyspnea and pain, whereas dyspnea and fatigue are the most troublesome in ILD patients (12,13). Furthermore, both respiratory and non-respiratory symptoms have been shown to be accentuated with increasing dyspnea severity (14). A previous study from our center demonstrated improvements in sleep, cough, and pain in LTx candidates, but differences between COPD and ILD were not evaluated and may have important management considerations (15).
In addition to a lack of comparison between symptoms in ILD and COPD patients, the integration of clinical measures such as oxygen requirements, exercise capacity and frequency of respiratory exacerbations has not been undertaken in the PC setting pre-LTx. Exercise capacity has been shown to be relatively preserved with pulmonary rehabilitation (16); however, respiratory exacerbations in ILD and COPD patients can lead to increased symptoms and accelerated lung function decline (17,18); furthermore, oxygen requirements typically increase in advanced lung disease, with a more significant increase among ILD patients (19). As symptoms have important effects on HRQL, exercise capacity, and healthcare utilization, a better understanding of symptom burden may aid with management in LTx candidates (20,21). Specifically, it may help inform pharmacological strategies and stratify which patients may be more likely to benefit from therapy.
The main aims of the study were: (I) to compare symptoms in ILD and COPD LTx candidates using the ESAS; (II) to assess the trajectory of change in ESAS domains in relation to pre-LTx exercise capacity, oxygen requirements, and respiratory exacerbations. We hypothesized that both groups will derive symptomatic benefit from PC support, but ILD patients will report a higher symptom burden compared to COPD patients. Furthermore, ILD LTx candidates will have greater progression of their symptoms due to increased oxygen requirements and more difficulty with recovery post-ILD exacerbations. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-905/rc).
Methods
Study design and patient population
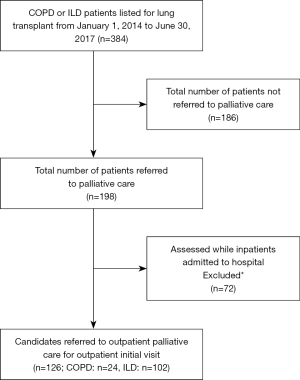
This was a retrospective cohort study of consecutive adult LTx candidates listed between January 1, 2014 to June 30, 2017 with a primary diagnosis of ILD or COPD, and were assessed in the Toronto Transplant Palliative Care Clinic (TPCC), located at the University Health Network (UHN). LTx candidates listed for a re-transplantation or those admitted at the time of initial PC assessment were excluded (Figure 1). A convenience sample was chosen due to the availability of patients within a pre-specified database. All missing data from the original database was identified by the authors and a manual chart review was conducted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the UHN Research Ethics Board (REB # 18-5279). Individual consent for this retrospective analysis was waived.
Toronto transplant PC clinic
The TPCC provides a multimodal approach and includes non-pharmacological strategies to improve HRQL, as well as pharmacologic recommendations to ease symptoms (22). At our center, not all patients are referred to PC. Patients are referred to PC based at the discretion of the LTx program healthcare provider. The initial outpatient TPCC visit typically involves a consultation with a PC physician for comprehensive evaluation of physical and psychosocial symptoms, and advance care planning. Follow-up typically occurs at intervals of 4–6 weeks during the pre-LTx period depending on patient needs and symptom severity (22). Low dose oral opioids are often prescribed as needed for management of dyspnea or cough (22). Anxiolytics (i.e., benzodiazepines) and sleep aids are used to assist with anxiety and sleep.
The type of opioid and dose (mg) were abstracted from chart review and converted to morphine equivalents. The number of TPCC visits that occurred in the pre-LTx period were determined from the TPCC clinical database (15).
Pre-transplant rehabilitation program
All LTx candidates participated in a mandatory, supervised outpatient exercise training program three times per week during the study period (16). The exercise sessions were comprised of aerobic, resistance and flexibility training with progression and intensity previously described (19). In addition, LTx candidates underwent nutritional assessment by a dietitian prior to listing and were provided with dietary counselling at our center as needed (23).
Edmonton symptom assessment score
The ESAS is an 11-point numerical score (0= no symptoms, 10= worst symptoms), and includes twelve symptom domains (e.g., fatigue, dyspnea, sleep, depression, etc.) (11). The additional symptom domain of cough was added to the ESAS tool due to its prevalence in lung disease. The ESAS allows assessment of symptoms over time and the ESAS scores (individual domains and total score) with each PC visit were abstracted. The minimally important difference (MID) for each ESAS domain has been described in the oncology field, with 1-point per domain often used (24). ESAS has also been used to measure symptoms in both ambulatory COPD and ILD patients (13,14,25).
Clinical measurements
The following were abstracted: age, sex, anthropometric measurements [weight (kg), height (cm), body mass index (BMI, kg/m2)], and primary respiratory diagnosis (ILD or COPD) were taken at listing. Lung Allocation Score and Canadian listing urgency status (status 1, 2, or rapidly deteriorating) were also assessed at the time of listing (26). The Canadian Listing Urgency is a global measure of disease severity used in Canadian centres, which has been shown to have moderate association with Lung Allocation Score (LAS) (26). We also evaluated whether patients were delisted or died pre-LTx.
Physiological parameters
Exercise capacity was evaluated using six-minute walk distance (6MWD, in metres) as per American Thoracic Society (ATS) standards (27). This is performed at initial LTx assessment, at the time of LTx listing, and every three months while awaiting transplantation (16). Exertional oxygen requirements (liters per minute), were abstracted and converted to the fraction of inspired oxygen (FiO2) from 6MWD reports (16). Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) at the time of LTx listing were abstracted from chart review, which were performed in the pulmonary function lab as per ATS standards (28).
Respiratory exacerbations
The number of hospital admissions for respiratory exacerbations between LTx listing and transplantation were quantified from medical records. We characterized an exacerbation from chart review on the basis of hospital admission (with overnight stay) for acute deterioration in respiratory status requiring antibiotics or corticosteroids. We also included respiratory exacerbations at other facilities if they were documented in the chart.
Statistical analysis
Descriptive analyses of the variables were expressed as mean ± standard deviation, proportions, or median with interquartile ranges (IQRs). Differences in patient characteristics between ILD and COPD LTx candidates at baseline were reported using the 95% confidence interval (CI). T-tests, chi-squared tests and Mann-Whitney U tests were used to compare clinical parameters and ESAS scores between ILD and COPD patients at baseline and longitudinally. All normally distributed data are presented by the mean and standard deviation (± SD). Non-normally distributed data are presented by the median and IQR. We did not use imputation methods to control for confounding. Sensitivity analyses were performed for the various subgroups and these can be found within Supplementary Appendix. A P value of <0.05 was considered to be statistically significant. Analysis was performed using GraphPad Prism (version 9.2.0).
Results
Study population
A total of 384 patients were listed for LTx during the study period (June 1, 2014 to June 30, 2017), as shown in Figure 1. A total of 186 patients listed for LTx during the study period were not referred to TPCC and 72 LTx candidates were excluded as they were assessed by PC as inpatients (baseline characteristics for excluded groups in Table S1). The cohort was comprised of 102 ILD and 24 COPD patients assessed in the TPCC during the study period (Table 1). ILD patients were older (62±8 vs. 57±7 years; P=0.01), had a higher listing BMI, and LTx urgency reflected by both the Canadian listing status and the LAS (Table 1). ILD patients were observed to have greater 6MWD and oxygen requirements at baseline compared to COPD. ILD and COPD LTx candidates had similar wait times pre-LTx [median days of 153 (IQR, 76–294) and 167 (IQR, 112–381), in patients with ILD and COPD, respectively, P=0.17] and median number of TPCC visits [ILD 2 (IQR, 1–3) vs. COPD 2 (IQR, 1–2), P=0.11]. There was no significant difference in time from LTx listing to first PC visit (P=0.79). A diagnosis of ILD was more likely to be associated with referral to PC (OR =2.8, 95% CI: 1.8–4.5, P<0.001).
Table 1
| Parameter | Total (n=126) | ILD (n=102) | COPD (n=24) | P value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 61±8 | 62±8 | 57±7 | 0.01 |
| Male sex (%) | 59 (47%) | 50 (49%) | 9 (38%) | 0.31 |
| Body mass index (kg/m2, mean ± SD) | 26±4 | 26±4 | 24±5 | 0.003 |
| Lung allocation score (n=112) | 42 [34–45] | 39 [35–48] | 33 [32–35] | <0.001 |
| Status at listing (n=126) | ||||
| Status 1 | 60 (48%) | 41 (40%) | 19 (79%) | 0.003 |
| Status 2 | 62 (49%) | 57 (56%) | 5 (21%) | |
| Status 3 | 4 (3%) | 4 (4%) | 0 | |
| Time from transplant listing to first PC visit (days) | 38 [12–108] | 37 [12–98] | 89 [10–139] | 0.79 |
| FVC (% predicted; n=102) | 48±15 | 46±12 | – | N/A |
| FEV1 (% predicted; n=23) | 43±16 | – | 22±8 | |
| 6MWD at listing (m; n=122) | 330 [252–404] | 337 [245–407] | 277 [245–324] | 0.04 |
| 6MWD at listing (% predicted; n=122) | 61 [48–81] | 66 [48–84] | 52 [49–61] | 0.02 |
| Oxygen use at listing (patients) | 115 | 95 | 20 | 0.23 |
| Oxygen use at listing† (FiO2; n=122) | 0.48 [0.3–0.5] | 0.52 [0.3–0.6] | 0.30 [0.3–0.4] | < 0.001 |
| BiPAP or CPAP use at listing | 8 | 5 | 3 | 0.18 |
| ≥1 admission to hospital‡ | 41 (33%) | 35 (34%) | 6 (25%) | 0.47 |
| Median length of stay, days | 6 [4–11] | 7 [4–12] | 4 [2–15] | 0.17 |
Data presented as mean ± SD, median [Interquartile range], and n (%). Missing data is reflected by numbers available for the row. †, FiO2 calculated based upon standard conversions (14). ‡, admissions to hospital include admissions to our centre, or another site, for respiratory exacerbations as documented in the chart. Range of admissions =1–4. BiPAP, bilevel positive airway pressure; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FiO2, fraction of inspired oxygen; ILD, interstitial lung disease; PC, palliative care; SD, standard deviation; 6MWD, 6-minute walk distance.
Symptoms and opioid prescription in ILD and COPD transplant candidates
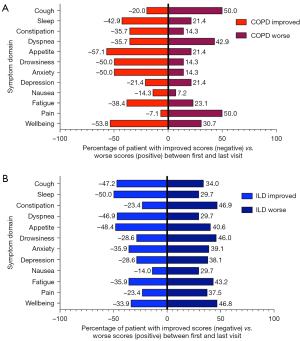
The most troublesome symptoms reported by both ILD and COPD patients during the first TPCC visit included dyspnea [median score of 8 (IQR, 6–9), cough 7 (IQR, 3–8), and fatigue 6 (IQR, 4–8)]. ILD patients reported higher cough scores [7 (IQR, 4–9) vs. 4 (IQR 1–7), P<0.001], but had less drowsiness [2 (IQR, 0–4) vs. 3 (IQR 1–5), P=0.04] than COPD LTx candidates (Figure 2). While the absolute changes in ESAS by domain were not statistically significant between ILD and COPD patients, the improvement in anxiety and sleep exceeded the MID (MID ≥1), as did worsening constipation and drowsiness amongst ILD patients. COPD patients described worsening pain that exceeded the MID, while awaiting LTx (Figure S1). We noted significant individual variability in COPD (Figure 3A) and ILD patients (Figure 3B) with symptom improvement and worsening in both groups. Both ILD and COPD patients experienced a comparable change in each direction (i.e., improvement or worsening) in each individual symptom domain, with the magnitude of change generally ≤3 ESAS points. Over the course of TPCC visits, total symptom burden in ILD patients progressively increased, while symptoms in COPD patients remained stable in the pre-LTx period (Figure S2).

After the initial PC visit, a similar proportion of ILD and COPD patients were prescribed opioids, 91.3% and 92.3%, respectively. COPD patients generally remained on as needed opioid dosing, whereas a greater proportion of ILD patients were using higher doses of opioids (Figure S3).
Exercise capacity and supplemental oxygen use
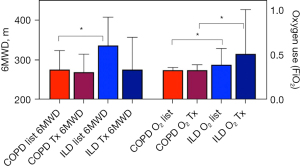
ILD patients had a higher 6MWD at listing [337 m (IQR, 245–407) vs. 277 m (IQR, 245–324); P=0.04; Table 1], and had a greater 6MWD decline in the pre-LTx period compared to COPD patients [–47 m (IQR, −99 to −5) vs. −8 m (IQR, −50 to 29), P=0.006]. ILD patients had higher supplemental oxygen use at baseline and last assessment pre-LTx (Figure 4). When the median values for listing 6MWD and oxygen requirements were utilized to establish two groups (equal/above and below the median), there was no difference observed in baseline ESAS scores or their change in cough, dyspnea or fatigue, the three most symptomatic domains for LTx candidates (Figure S4).
There were 41 (33%) patients admitted to hospital at least once in the pre-LTx period for a respiratory exacerbation, with no difference in the number of hospital admissions between ILD and COPD LTx candidates during the pre-LTx period (Table 1). When stratified by ≥1 respiratory exacerbations compared to no exacerbations, total ESAS and the domain scores for dyspnea, cough and fatigue were independent of respiratory exacerbation history (Figure S4).
ESAS Scores in LTx candidates who were delisted or died pre-transplant
There were 12 (10%) ILD and 2 (2%) COPD candidates who were delisted or died pre-LTx. The 2 patients with COPD were delisted due to malnutrition and lung cancer. Of the 10 patients with ILD, 7 died due to progression or exacerbation, 2 were delisted due to new diagnosis of lung cancer, and 3 were delisted or died for unknown reasons. ILD candidates who were delisted or died reported higher depression [4.5 (IQR, 0–7) vs. 1 (IQR, 0–3) P=0.04], anxiety [5.5 (IQR, 3–8) vs. 2 (IQR, 0–5), P=0.01], and dyspnea scores [9.5 (IQR, 7–10) vs. 8 (IQR, 6–9), P=0.01] compared to ILD patients who were transplanted (Table S2). Similarly, the two COPD patients who were delisted or died also had higher ESAS scores (≥1 point difference) in the domains of fatigue, depression, anxiety, drowsiness, wellbeing, constipation, sleep, and cough, but given the small sample size no statistical evaluation was undertaken (Table S3).
Discussion
This is the first study to compare symptoms and disease trajectory of ILD and COPD LTx candidates while awaiting lung transplantation. We observed that ILD patients had increased cough and less drowsiness at the first PC clinic visit, but otherwise symptoms were similar to COPD patients during the pre-LTx period. We did not observe an association between the change in ESAS domains and disease severity markers such as 6MWD, oxygen requirements, or respiratory exacerbation history. Dyspnea and mental health domain scores (anxiety and depression) were significantly worse in ILD patients who were medically delisted or died pre-LTx.
Both ILD and COPD LTx candidates had a significant symptom burden with the most troublesome symptoms being dyspnea, cough and fatigue. Cough was worse in patients with ILD, whereas they had lower drowsiness scores compared to COPD patients. Our findings are in line with previous work from our centre, which demonstrated increased symptoms experienced by LTx candidates including dyspnea (median 7/10), tiredness (6/10), cough (5/10) and fatigue (5/10) (15,22). Dyspnea is troublesome amongst both ILD and COPD LTx candidates, and has been associated with worse HRQL, limitations in activities of daily living, and wellbeing (29-31). A previous ILD study has shown that over 80% of patients reported cough, and that it was associated with decreased time to LTx and increased mortality, independent of disease severity (32). Thus, cough may not only be a troublesome symptom for patients, but may also be a surrogate marker of morbidity and mortality, independent of other physiologic limitations, but requires further evaluation. Management of cough is often multi-faceted utilizing low dose opioids in the PC setting and addressing other contributors of cough such as gastroesophageal reflux symptoms and post-nasal drip (33). Furthermore, management of cough and dyspnea can help alleviate fatigue in both ILD and COPD patients (34,35).
We sought to measure the symptom trajectory among COPD and ILD patients in the pre-LTx period. We used a MID of 1-point for each ESAS domain, which has been previously applied in the oncology field (24). COPD patients reported worsening pain exceeding the MID of 1 point. This is in keeping with the literature as COPD patients are more likely to have chronic pain and utilize opioids or other pain-related medications compared to patients with other chronic disorders (36). Thoracic pain, may be partly caused by osteoporotic vertebral deformity, and costotransverse and intervertebral arthropathy in patients living with COPD, resulting in pain that is most often reported in the trunk (37,38). A cycle of pain that disrupts sleep or aggravates psychosocial symptoms may lead to worsening dyspnea and upper body pain (36,39). Furthermore, sleep was improved in ILD patients who comprised the majority of our cohort, but the lack of improvement in COPD patients remains to be determined. Our findings are in line with previous work showing that sleep improved by about 2 points in LTx candidates (15). COPD is often associated with sleep disturbances and daytime sleepiness, which may be in part due to hypoxemia, obstructive apneas, and possibly pain, which may contribute to greater sleep fragmentation and poor quality of sleep (40,41). Thus, attention to pain and sleep disordered breathing are important considerations in the pre-LTx period, especially in COPD patients (36-38,41).
We did not see an association between the trajectory of disease severity markers and ESAS scores. Functional measures such as 6MWD, oxygen saturation at baseline, Borg dyspnea scores, and comorbidities have been shown to be similar between ILD and COPD patients (42). Exertional oxygen desaturation has been shown to be more profound in ILD patients compared to COPD patients, accompanied by greater exertional desaturation among ILD patients awaiting LTx (19,42). While ILD patients had more physiologically advanced disease compared to COPD patients, their ESAS scores were similar. This is likely attributable to the multifactorial mechanisms of dyspnea in COPD patients beyond hypoxemia which includes excessive inspiratory neuronal drive to breathe, and skeletal and diaphragmatic muscle dysfunction (43). This further serves to highlight the other key roles of PC involvement, such as advanced care planning and psychosocial support, as complementary to exercise training and provided by the pulmonary rehab program (15,43).
Though we expected that ILD patients would experience a greater decline in 6MWD and increase in oxygen requirements coinciding with increased ESAS scores, symptom stability was observed. Similarly, irrespective of exacerbation history (≥1 exacerbations versus no exacerbations), ESAS scores were similar in both groups at baseline and longitudinally independent of exacerbations. We suspect that this stabilization in symptoms may be in part due to the multidisciplinary care LTx candidates receive. All LTx candidates participate in a standard structured pulmonary rehabilitation program at our center. LTx candidates also have access to nutritional support, which can attenuate cachexia and improve general well-being and breathlessness in advanced lung disease (23,44).
Furthermore, 94% of LTx candidates received opioids after their first PC clinic visit, which has been shown to improve dyspnea, cough, and HRQL in advanced lung disease, as supported by several international guidelines (45). This is comparable to previously reported opioid prescribing patterns by PC at our centre (15). Despite these recommendations, very few patients were on opioids prior to their referral to the TPCC. At other centres, opioid prescription in the pretransplant period has been reported as approximately 15%, and did not increase the risk of death or retransplantation (46). Some of the hesitation to prescribe opioids in the pretransplant period may be related to perceived concerns of safety (47,48). At our centre, the possible harms of opioid prescription are safeguarded by the involvement of a multidisciplinary team of transplant, PC and pulmonary rehabilitation physicians and allied health care team members. While we do not routinely collect blood gas data on our lung transplant population for opioid prescribing, it is possible that concerns for hypercapnia contributed to a lower opioid dose prescribed to the COPD patients. Nevertheless, the current study highlights that one-third of ILD and COPD patients were referred to the TPCC clinic, which illustrates the emphasis of PC supports in the Toronto Lung Transplant Program.
While both COPD and ILD groups were similar at baseline, there were more women in the group referred to PC. It has been shown previously that women report more symptoms and symptom distress, when compared to men (49,50). This may be because women need to report higher symptom burden levels for this to be documented by their providers (49). This is an interesting observation, which may indicate the need for more careful referral practices to mitigate potential gender bias.
Despite recent advances in pharmacotherapy, patients with advanced ILD are at a higher risk of delisting or mortality compared to other indications for lung transplantation (51). In our study, over ten percent of ILD patients who were referred for PC support were either de-listed or died pre-LTx. Compared to those who were transplanted, ILD candidates who were delisted or died pre-LTx reported higher rates of depression, anxiety, impaired wellbeing and dyspnea. It has been shown that psychosocial ESAS symptoms have the tendency to start out mild in the two-year prior to death in ILD, but increase precipitously with disease progression (13). While there was significant individual variability, there was a higher progressive symptom burden amongst ILD patients who were delisted or died pre-LTx. Furthermore, timely PC referral is important when patients first become symptomatic and concurrently while pursuing lung transplantation, as emphasized by PC guidelines (10).
Limitations
Our study has several limitations. First, we were unable to assess the individual contribution of PC interventions on symptoms compared to pre-LTx rehabilitation, as all patients were participating in standard supervised rehabilitation at our center. Secondly, self-reported data on symptoms using the ESAS has not been validated in this population, but are frequently utilized at our center to guide clinical management in PC and also applied in non-listed COPD and ILD patients at other centers (13,15,22,25). Thirdly, the reporting of admission secondary to respiratory exacerbations was ascertained from chart review, but it’s possible that exacerbations occurring outside of our institution may have been missed. In addition, our study was not powered to assess mortality, as events such as medical delisting or death were not common. Given the retrospective nature of our study, we did not have HRQL measures or activities of daily living, which could have provided another opportunity to compare symptom burden to daily function in this cohort. We also recognize that the referral process for PC at our center is at the discretion of the LTx team, thus the cohort of ILD and COPD patients followed at our center may be different from other LTx programs. Because referrals are made by the LTx team, we recognize that this may have introduced an element of referral bias. Our primary objective was to compare patients with COPD to patients with ILD, and both groups may represent a more symptomatic group compared to those who were not referred to PC. Ideally, all patients awaiting lung transplantation could benefit from PC referral. If it is not possible to refer all patients, then a screening tool for assessing symptom burden may be applied to all patients to determine those who are most symptomatic to maximize benefit of PC. Prospective studies would be necessary to compare patients who are referred to PC versus those not referred to directly determine the effect of PC interventions.
Conclusions
The present study highlights that ILD patients had higher exercise capacity and higher oxygen requirements at baseline than patients with COPD, and experienced greater decline in exercise capacity while on the LTx list. Nevertheless, ESAS scores at baseline and follow-up amongst ILD patients were comparable with COPD patients, illustrating the discordance between symptoms and disease severity measures. Despite this, it is important for providers not to dismiss symptoms of COPD patients considering less severe disease physiology. Patients with ILD were more likely to be delisted or die pre-LTx, and report a higher symptom burden, highlighting the importance of PC in this population. There is increasing support for the role of PC to aid in symptom management in the pre-transplantation period and we suspect that this will only become more important in the future (52,53). The ESAS has a role in identifying severe symptom burden in those with advanced lung disease on the LTx waiting list and may serve as a complement to traditional markers of disease severity. In light of the discordance between symptoms and disease severity, future directions may involve referral of all patients to PC, which was recently implemented at our centre. Given the complex interplay of PC and multi-disciplinary support in LTx candidates, future randomized controlled trials of PC interventions would be helpful to determine their effect on symptoms and functional measures in this population.
Acknowledgments
The authors would like to acknowledge the support of the Toronto Lung Transplant Program and Mr. Ramraj Rajakumar and Mr. Svetolik Kovacevic for their help with data abstraction from the OTTR Database.
Funding: Dmitry Rozenberg receives support from the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-905/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-905/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-905/coif). Dmitry Rozenberg receives financial support from the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University Health Network Research Ethics Board (REB # 18-5279). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022;10:447-58. [Crossref] [PubMed]
- Kaul B, Cottin V, Collard HR, et al. Variability in Global Prevalence of Interstitial Lung Disease. Front Med (Lausanne) 2021;8:751181. [Crossref] [PubMed]
- Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40:1060-72. [Crossref] [PubMed]
- Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021;40:1349-79. [Crossref] [PubMed]
- Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med 2013;34:421-30. [Crossref] [PubMed]
- Vermuelen KM, van der Bij W, Erasmus ME, et al. Long-term health-related quality of life after lung transplantation: different predictors for different dimensions. J Heart Lung Transplant 2007;26:188-93. [Crossref] [PubMed]
- Colman R, Singer LG, Barua R, et al. Characteristics, interventions, and outcomes of lung transplant recipients co-managed with palliative care. J Palliat Med 2015;18:266-9. [Crossref] [PubMed]
- Singer JP, Chen J, Katz PP, et al. Defining novel health-related quality of life domains in lung transplantation: a qualitative analysis. Qual Life Res 2015;24:1521-33. [Crossref] [PubMed]
- Mahler DA, Selecky PA, Harrod CG. Management of dyspnea in patients with advanced lung or heart disease: practical guidance from the American college of chest physicians consensus statement. Pol Arch Med Wewn 2010;120:160-6. [Crossref] [PubMed]
- Lanken PN, Terry PB, Delisser HM, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912-27. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [Crossref] [PubMed]
- Antoniu SA, Apostol A, Boiculese LV. Extra-respiratory symptoms in patients hospitalized for a COPD exacerbation: Prevalence, clinical burden and their impact on functional status. Clin Respir J 2019;13:735-40. [Crossref] [PubMed]
- Rajala K, Lehto JT, Sutinen E, et al. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med 2018;18:172. [Crossref] [PubMed]
- Rantala HA, Leivo-Korpela S, Lehto JT, et al. Dyspnea on Exercise Is Associated with Overall Symptom Burden in Patients with Chronic Respiratory Insufficiency. Palliat Med Rep 2021;2:48-53. [Crossref] [PubMed]
- Freeman N, Le LW, Singer LG, et al. Impact of a transplant palliative care clinic on symptoms for patients awaiting lung transplantation. J Heart Lung Transplant 2016;35:1037-9. [Crossref] [PubMed]
- Li M, Mathur S, Chowdhury NA, et al. Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant 2013;32:626-32. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [Crossref] [PubMed]
- Wickerson L, Brooks D, Reid WD, et al. Exertional Oxygen Requirements During Exercise Training in Advanced Interstitial Lung Disease. J Cardiopulm Rehabil Prev 2018;38:419-24. [Crossref] [PubMed]
- Byng D, Lutter JI, Wacker ME, et al. Determinants of healthcare utilization and costs in COPD patients: first longitudinal results from the German COPD cohort COSYCONET. Int J Chron Obstruct Pulmon Dis 2019;14:1423-39. [Crossref] [PubMed]
- Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res 2017;18:67. [Crossref] [PubMed]
- Wentlandt K, Dall'Osto A, Freeman N, et al. The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant 2016;30:1591-6. [Crossref] [PubMed]
- Chohan K, Park J, Dales S, et al. Evaluation of Malnutrition Risk in Lung Transplant Candidates Using the Nutritional Risk Index. Transplant Direct 2020;6:e574. [Crossref] [PubMed]
- Hui D, Shamieh O, Paiva CE, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage 2016;51:262-9. [Crossref] [PubMed]
- Elbehairy AF, McIsaac H, Hill E, et al. Impact of a Specialized Ambulatory Clinic on Refractory Breathlessness in Subjects With Advanced COPD. Respir Care 2020;65:444-54. [Crossref] [PubMed]
- Hirji A, Zhao H, Ospina MB, et al. Clinical judgment versus lung allocation score in predicting lung transplant waitlist mortality. Clin Transplant 2020;34:e13870. [Crossref] [PubMed]
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428-46. [Crossref] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-e88. [Crossref] [PubMed]
- Lutogniewska W, Jastrzebski D, Wyrwol J, et al. Dyspnea and quality of life in patients referred for lung transplantation. Eur J Med Res 2010;15:76-8. [Crossref] [PubMed]
- Swigris JJ, Kuschner WG, Jacobs SS, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax 2005;60:588-94. [Crossref] [PubMed]
- Ryerson CJ, Donesky D, Pantilat SZ, et al. Dyspnea in idiopathic pulmonary fibrosis: a systematic review. J Pain Symptom Manage 2012;43:771-82. [Crossref] [PubMed]
- Key AL, Holt K, Hamilton A, et al. Objective cough frequency in Idiopathic Pulmonary Fibrosis. Cough 2010;6:4. [Crossref] [PubMed]
- van Manen MJ, Birring SS, Vancheri C, et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev 2016;25:278-86. [Crossref] [PubMed]
- Kahlmann V, Moor CC, Wijsenbeek MS. Managing Fatigue in Patients With Interstitial Lung Disease. Chest 2020;158:2026-33. [Crossref] [PubMed]
- Booth S, Johnson MJ. Improving the quality of life of people with advanced respiratory disease and severe breathlessness. Breathe (Sheff) 2019;15:198-215. [Crossref] [PubMed]
- Roberts MH, Mapel DW, Hartry A, et al. Chronic pain and pain medication use in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2013;10:290-8. [Crossref] [PubMed]
- Chen YW, Camp PG, Coxson HO, et al. Comorbidities That Cause Pain and the Contributors to Pain in Individuals With Chronic Obstructive Pulmonary Disease. Arch Phys Med Rehabil 2017;98:1535-43. [Crossref] [PubMed]
- Chen YW, Coxson HO, Coupal TM, et al. The contribution of thoracic vertebral deformity and arthropathy to trunk pain in patients with chronic obstructive pulmonary disease (COPD). Respir Med 2018;137:115-22. [Crossref] [PubMed]
- Lohne V, Heer HC, Andersen M, et al. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung 2010;39:226-34. [Crossref] [PubMed]
- Lal C, Kumbhare S, Strange C. Prevalence of self-reported sleep problems amongst adults with obstructive airway disease in the NHANES cohort in the United States. Sleep Breath 2020;24:985-93. [Crossref] [PubMed]
- Shawon MS, Perret JL, Senaratna CV, et al. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med Rev 2017;32:58-68. [Crossref] [PubMed]
- Du Plessis JP, Fernandes S, Jamal R, et al. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology 2018;23:392-8. [Crossref] [PubMed]
- O'Donnell DE, Milne KM, James MD, et al. Dyspnea in COPD: New Mechanistic Insights and Management Implications. Adv Ther 2020;37:41-60. [Crossref] [PubMed]
- Efthimiou J, Fleming J, Gomes C, et al. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988;137:1075-82. [Crossref] [PubMed]
- Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2019;3:210-32. [Crossref]
- Vahidy S, Li D, Hirji A, et al. Pretransplant Opioid Use and Survival After Lung Transplantation. Transplantation 2020;104:1720-5. [Crossref] [PubMed]
- Bloom CI, Slaich B, Morales DR, et al. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J 2018;51:1701879. [Crossref] [PubMed]
- Bajwah S, Yorke J. Palliative care and interstitial lung disease. Curr Opin Support Palliat Care 2017;11:141-6. [Crossref] [PubMed]
- Falk H, Henoch I, Ozanne A, et al. Differences in Symptom Distress Based on Gender and Palliative Care Designation Among Hospitalized Patients. J Nurs Scholarsh 2016;48:569-76. [Crossref] [PubMed]
- O'Neill ES, Morrow LL. The symptom experience of women with chronic illness. J Adv Nurs 2001;33:257-68. [Crossref] [PubMed]
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant 2019;19:404-84. [Crossref] [PubMed]
- Pawlow PC, Blumenthal NP, Christie JD, et al. The palliative care needs of lung transplant candidates. Clin Transplant 2020;34:e14092. [Crossref] [PubMed]
- Scully BB, Nolley EP, Bush EL. Palliative care in lung transplantation. Ann Palliat Med 2022;11:927-35. [Crossref] [PubMed]


