
Effect of exercise on cardiovascular risk in sedentary postmenopausal women: a systematic review and meta-analysis
Highlight box
Key findings
• Physical exercise not only increases oxygen consumption, but also reduces CVD-related risk factors.
What is known and what is new?
• CVD is currently the leading cause of death in China. Postmenopausal women face a higher risk of CVD.
• In the present study we performed a meta-analysis to systematically analyze all related studies to come to an accurate conclusion.
What is the implication, and what should change now?
• The findings suggest that studies are needed into the effect of exercise on CVD risk factors in sedentary postmenopausal women and that postmenopausal women should undertake regular physical exercise.
Introduction
Cardiovascular disease (CVD) is currently the leading cause of death in China (1). With age, metabolic abnormalities such as obesity, dyslipidemia, hypertension, and abnormal blood glucose become important risk factors for CVD (2) in both men and women (3). However, the risk of vascular dysfunction increases markedly in postmenopausal women because of estrogen deficiency, and so they face a higher risk of CVD (4,5). For example, compared with premenopausal women, postmenopausal women have increased arterial stiffness, and a daily lack of physical exercise places them at increased risk of CVD (3,6). In the US, approximately 40% of adult women (25 million) are postmenopausal, so finding effective methods to reduce CVD risk in this population is of significance in terms of both lowering the healthcare burden and improving national health (7).
The American College of Sports Medicine has stated that the amount of physical exercise is related to the risk of developing various diseases, including CVD (8,9), and a lack of exercise is a well-known independent risk factor for CVD (10). Many studies have shown that moderate to low intensity aerobic exercise can help improve people’s cardiovascular function, thereby reducing their risk of CVD (11,12). Such regular intervention can improve CVD-related metabolic syndrome presenting with abnormal blood pressure, lipid, and glucose levels (13). Due to estrogen depletion and metabolic alterations, postmenopausal women are at substantially increased risk for CVD; However, unfortunately, only 30% of postmenopausal women exercise regularly (9). In addition, a study confirmed that long-term aerobic exercise significantly decreased adiposity levels in previously sedentary postmenopausal women, which was significantly associated with the development of cardiovascular disease (14). At present, although a study has reported on the effects of physical activity on CVD-related risk factors in postmenopausal women, no systematic conclusion has been reached (15). Thus, in the present study we performed a meta-analysis to systematically analyze all related studies to come to an accurate conclusion. We present the following article in accordance with the PRISMA-P reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1395/rc).
Methods
Search strategy
Literature searches were conducted on the Web of Science, Cochrane, PubMed, Chinese National Knowledge Infrastructure, and WanFang databases for articles published. The keywords used were “Exercise”, “Sedentary”, and “Cardiovascular risk”.
Inclusion criteria
To be eligible for inclusion in the meta-analysis, articles had to meet the following criteria:
- Study subjects: all subjects had to postmenopausal (i.e., no menstrual period for at least 12 consecutive months), elderly (age >60 years), sedentary for long periods of time and without regular exercise for at least 1 year;
- Intervention measures: subjects were divided into an exercise group and a non-exercise group; the exercise group undertook routine aerobic physical exercise for at least 30 minutes per week according to the plan created by the investigators, whereas the non-exercise group did not participate in physical exercise;
- Study design: studies had to be randomized controlled trials (RCT) published in relevant medical journals in China and internationally;
- Outcome measures: studies had to report on at least 1 of the following measures: heart rate (HR), oxygen consumption (Vo2), diastolic blood pressure (DBP), systolic blood pressure (SBP), triglycerides (TG), cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), body mass index (BMI), waist circumference, and glucose;
- Study subjects (or their family members) must have provided informed consent.
Exclusion criteria
Studies were excluded if they met the following criteria:
- Study subjects: current smokers, patients with CVD, renal disease, or thyroid disease, patients currently receiving hormone replacement therapy, patients who had undertaken a weight loss diet in the previous year, and patients with mental illness or cognitive impairment;
- Qualitative studies, secondary studies, systematic reviews, meta-analyses, animal experiments, and studies for which the full text was not available.
Literature screening and data extraction
The retrieved studies were imported into Endnote 7.0 (Stamford, Connecticut, USA), and duplicates were removed. Both authors independently screened the literature based on the inclusion and exclusion criteria, and extracted and organized the data (including title, author, study design, study subject, intervention measures, and outcome measures).
Literature quality assessment
The Cochrane Collaboration’s tool for assessing risk of bias was used to assess the quality of the included studies, which mainly included six aspects: (I) selection bias, including whether the generation and allocation of random sequences were hidden; (II) implementation bias: whether the study patients and researchers were blinded; (III) measurement bias: whether the outcome was evaluated in a blinded manner; (IV) follow-up bias: whether the outcome data were missing; (V) reporting bias: whether there was selective reporting; (VI) other biases. The analysis results are shown in Figure 1 and Figure 2.
Statistical analysis
The meta-analysis was performed using Stata16.0. (IBM Corp., Armonk, NY, USA) Continuous variable outcomes are presented as the standardized mean difference (SMD) with 95% confidence intervals (CIs), whereas categorical variable outcomes are presented as odds ratios (ORs) and 95% CIs. Heterogeneity was tested using a Chi-squared test and I2 statistics, with the α level set to 0.05. If I2<50% and P>0.05, there was no statistical heterogeneity across studies and a fixed-effects model was used to combine the effect size; otherwise, the random-effects model shall be adopted, and the sensitivity analysis shall be conducted for the possible causes of heterogeneity. Sensitivity analysis was performed to test the stability of the findings. Funnel plots and egger’s test were used to assess publication bias. Differences were considered statistically significant at 2-sided P<0.05.
Results
Literature search results
From the 623 articles initially retrieved, 257 duplicates were excluded. A further 209 articles were excluded after screening the titles and abstracts and 143 articles were excluded after reading the full text and reported data. Finally, 14 studies were included in the meta-analysis (16-29). The literature screening process is shown in Figure 3. The 14 studies in the meta-analysis included 473 sedentary postmenopausal women, 250 in the exercise group and 223 in the non-exercise group. The characteristics of the included studies are presented in Table 1.
Table 1
| First author (reference) | Year | No. participants | Age (years) | Baseline BMI (kg/m2) | Study design | Outcome measures | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Non-exercise | Exercise | Non-exercise | Exercise | Non-exercise | ||||||
| Rodrigo (16) | 2008 | 37 | 22 | 54.3±2.5 | 53.1±1.9 | – | – | RCT | (e) (j) (k) | ||
| Malandish (17) | 2020 | 16 | 15 | 53.36±3.98 | 53.00±3.26 | 29.67±5.15 | 30.29±6.58 | RCT | (b) | ||
| Gómez-Tomás (18) | 2018 | 18 | 20 | 70.89±4.42 | 70.45±5.44 | 28.72±4.48 | 30.16±5.57 | RCT | (f) (g) (h) (j) (k) | ||
| Wong (19) | 2019 | 10 | 10 | 54±1 | 55±1 | 32.5±1.0 | 32.5±1.3 | RCT | (a) (b) | ||
| Dalleck (20) | 2009 | 8 | 10 | 55.4±3.2 | 57.4±4.6 | 28.1±4.5 | 30.0±8.7 | RCT | (c) (d) (e) (f) (g) (h) (i) (j) (k) | ||
| Gerage (21) | 2013 | 15 | 14 | 65.5±5.0 | 66.2±4.1 | 23.9±2.9 | 25.1±3.4 | RCT | (a) (b) | ||
| Pekas (22) | 2020 | 57 | 44 | <75 | <78 | – | – | RCT | (c) (d) (j) (k) | ||
| Jeon (23) | 2018 | 8 | 8 | 59±1 | 58±2 | 27.2±1.9 | 26.1±1.2 | RCT | (a) (b) (d) (e) (f) (g) | ||
| Son (24) | 2021 | 18 | 17 | 68.2±1.6 | 68.2±1.4 | 26.7±3.2 | 27.1±1.4 | RCT | (a) (c) (c) (d) (e) (f) (h) (j) (k) | ||
| Lee (25) | 2012 | 8 | 8 | 54.75±2.76 | 54.25±2.91 | 25.13±1.63 | 25.19±1.71 | RCT | (a) (e) (j) (k) | ||
| Nishiwaki (26) | 2011 | 8 | 8 | 56.4±1 | 24.14 | RCT | (b) (c) (d) (i) | ||||
| Shen (27) | 2013 | 22 | 22 | 57.86±0.64 | 59.10±0.83 | 22.74±0.47 | 23.72±0.66 | RCT | (a) (e) (i) (j) (k) | ||
| Son (28) | 2017 | 10 | 10 | 76±5 | 74.7±2 | 22.77±0.7 | 24.05±0.2 | RCT | (a) (c) (d) (i) | ||
| Zhang (29) | 2019 | 15 | 15 | 53.2±3.5 | 53.0±3.1 | 28.2±3.9 | 26.9±0.9 | RCT | (a) (b) (c) (d) | ||
Age and BMI are presented as the mean ± SD. (a), BMI; (b), heart rate; (c), diastolic blood pressure; (d), systolic blood pressure; (e), glucose; (f), triglycerides; (g), cholesterol; (h), waist circumference; (i), oxygen consumption; (j), high-density lipoprotein; (k), low-density lipoprotein. BMI, body mass index; RCT, randomized controlled trial.
Meta-analysis results for cardiac indicators
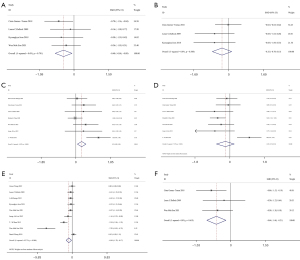
Six studies (17,19,21,23,26,29) compared HR between the exercise and non-exercise groups, 4 (20,26-28) compared Vo2 between the 2 groups, and 9 (20-26,28,29) compared DBP and SBP between the 2 groups. Random-effects models were used due to significant heterogeneity across studies (HR: I2=79.8%, P<0.001; Vo2: I2=69.6%, P=0.020; SBP: I2=77.1%, P<0.001; DBP: I2=64.2%, P=0.004). There was no significant difference in HR between the 2 groups (SMD =−0.11; 95% CI: −0.92 to 0.70; P=0.796; Figure 4A). The results showed that Vo2 was significantly higher in the exercise than non-exercise group (SMD =1.21; 95% CI: 0.38 to 2.05; P=0.004; Figure 4B), whereas DBP (SMD =−0.81; 95% CI −1.38 to −0.24; P=0.005; Figure 4C) and SBP (SMD =−0.98; 95% CI: −1.44 to −0.52; P=0.000; Figure 4D) were markedly lower in the exercise group.

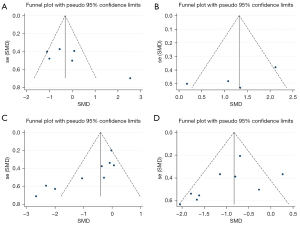
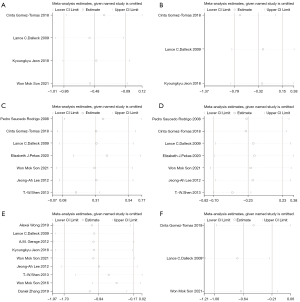
Sensitivity analysis was performed on the study results. There was no significant change in results after removing each study, one by one, indicating that the results of the meta-analysis were robust and reliable (Figure 5). Publication bias was also analyzed for the included studies. Diffuse distribution was observed on scatter plots representing DBP, whereas symmetrical funnel plots of the other cardiac indicators were found, indicating no publication bias (Figure 6; Egger’s test: HR: P=0.104; Vo2: P=0.225; DBP: P=0.005; SBP: P=0.170).
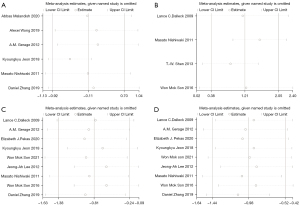
Meta-analysis results for obesity-related indicators
TG comparisons were reported in 4 studies (18,20,23,24), cholesterol comparisons were reported in 3 studies (18,20,23), HDL comparisons were reported in 7 studies (16,18,20,22,24,25,27), LDL comparisons were reported in 7 studies (16,18,20,22,24,25,27), BMI comparisons were reported in 9 studies (19-21,23-25,27-29), and waist circumference comparisons were reported in 3 studies (18,20,24). Fixed-effects models were used to analyze TG, cholesterol, HDL, and waist circumference (TG: I2=0.0%, P=0.791; cholesterol: I2=0.0%, P=0.388; HDL: I2=48.9%, P=0.068; waist circumference: I2=0.0%, P=0.619), whereas random-effects models were used to analyze LDL and BMI (LDL: I2=72.2%, P=0.001; BMI: I2=83.7%, P<0.001). Compared with the non-exercise group, the exercise group had significantly lower TG (SMD =−0.48; 95% CI: −0.86 to −0.09; P=0.016; Figure 7A). There were no marked differences in cholesterol (SMD =−0.32; 95% CI: −0.79 to 0.15; P=0.186; Figure 7B), significantly higher HDL (SMD =0.39; 95% CI: 0.04 to 0.73; P=0.027; Figure 7C), LDL (SMD =−0.23; 95% CI: −0.70 to 0.23; P=0.321; Figure 7D) between the 2 groups, BMI (SMD =−0.94; 95% CI: −1.70 to −0.17; P=0.016; Figure 7E), and waist circumference (SMD =−0.64; 95% CI: −1.06 to −0.21; P=0.003; Figure 7F).
In sensitivity analysis, there was no significant change in results after removing each study, one by one, indicating good stability (Figure 8). Publication bias was also analyzed for the included studies. Diffuse distribution was observed on scatter plots representing BMI, whereas symmetrical funnel plots of the other cardiac indicators were found, indicating no publication bias. (Figure 9; Egger’s test: TG: P=0.662; cholesterol: P=0.063; HDL: P=0.218; LDL: P=0.917; BMI: P=0.007; waist circumference: P=0.370).
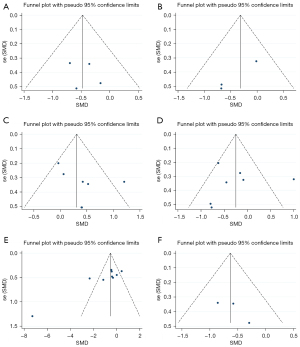
Meta-analysis results for blood glucose
Six studies (16,20,23-25,27) compared glucose between the exercise and non-exercise groups, and the random-effects model was used to combine the effect sizes due to significant heterogeneity among the studies (glucose: I2=77.6%, P<0.001). Glucose was found to be significantly lower in the exercise than non-exercise group (SMD =−1.03; 95% CI: −1.74 to −0.33; P=0.004; Figure 10A).

Further sensitivity analysis showed that there was no significant difference in the results after eliminating each study, one by one, indicating that the results of this study were stable (Figure 10B). Publication bias analysis was performed using a funnel plots, with the results showing basically symmetrically distribution on both sides of the funnel plot, indicating the absence of publication bias (Figure 10C; Egger’s test: P=0.412).
Discussion
This study explored the effects of physical exercise on risk factors for CVD in menopausal women. Fourteen RCTs were included in the meta-analysis after applying strict and exclusion criteria, with CVD-related outcome measures such as blood pressure, blood glucose, HR, and cholesterol. The data of various individual studies were combined through meta-analysis to enable a conclusion to be reached with high reliability. The combined results showed that postmenopausal women who undertook regular physical exercise had higher Vo2, and that both DBP and SBP were lower than in postmenopausal women who did not undertake physical exercise. Hypertension is a major risk factor for the development of CVD (30). The increased blood pressure in postmenopausal elderly women is attributed to the reduction in estrogen, increased sympathetic nerve activity, and decreased endothelial function (31). In addition, this could explain why the prevalence of hypertension is lower among premenopausal women than men of the same age group, but higher among postmenopausal women than men of the same age group (31). Physical exercise has considerable benefits in enhancing muscle strength and cardiovascular health, and increased muscle mass and strength are inversely associated with the risk of hypertension and frailty in older women (6,32,33).
Exercise has been proved to prevent age-related CVD risk factors (6,34). In the present meta-analysis, we found lower TG, BMI, and waist circumference and higher HDL in the exercise group, but no differences in cholesterol and LDL between the 2 groups. Optimal levels of LDL and HDL are <100 and >40 mg/dL, respectively (35). The lipid profile in menopausal women becomes abnormal due to changes in hormone levels, usually manifested as increased LDL and decreased HDL (36). This increase in LDL is thought to contribute to the increased risk of atherosclerosis and CVD (37). Consistent with the findings of the present study, Wooten et al. (38) and Ammar (39) reported that physical exercise was effective in reducing LDL levels in postmenopausal women. Obesity is often accompanied by increases in both BMI and waist circumference, leading to increased stiffness of the central and peripheral arteries (40). Abdominal obesity is a strong predictor of insulin resistance in older people (41), and reducing abdominal obesity and total fat mass can improve glucose levels in menopausal women (42). In the present study, glucose levels were lower in menopausal women who undertook physical exercise than in those who did not, which is similar to the findings of Conceição et al. (43). Collectively, physical exercise can comprehensively optimize the metabolic level of menopausal women and reduce the risk of CVD.
However, the present study has some limitations, such as the relatively small number of studies included and the small sample size. Further large-sample and multicenter RCTs are required to enhance the accuracy and reliability of the study results in order to provide guidance for the prevention of CVD in postmenopausal women.
Conclusions
Physical exercise not only increases Vo2, but also reduces CVD-related risk factors such as blood pressure, blood lipids, and BMI, in menopausal women. Thus, physical exercise is essential for reducing the risk of CVD and improving the quality of life in this population. Therefore, regular exercise, a beneficial lifestyle intervention, is recommended for postmenopausal elderly women to prevent CVD and promote physical performance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-P reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1395/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1395/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li JL. Exploring the effect of atmospheric ozone exposure on the risk of cardiovascular disease mortality based on a spatio-temporal Bayesian model. Guilin: Guilin University of Electronic Technology, 2021.
- Rochlani Y, Pothineni NV, Kovelamudi S, et al. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis 2017;11:215-25. [Crossref] [PubMed]
- Takahashi K, Miura S, Mori-Abe A, et al. Impact of menopause on the augmentation of arterial stiffness with aging. Gynecol Obstet Invest 2005;60:162-6. [Crossref] [PubMed]
- Hildreth KL, Ozemek C, Kohrt WM, et al. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause 2018;25:1011-9. [Crossref] [PubMed]
- Yeasmin N, Akhter QS, Mahmuda S, et al. Association of Hypertension with Serum Estrogen Level in Postmenopausal Women. Mymensingh Med J 2017;26:635-41. [PubMed]
- Figueroa A, Park SY, Seo DY, et al. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011;18:980-4. [Crossref] [PubMed]
- Morss GM, Jordan AN, Skinner JS, et al. Dose-response to exercise in women aged 45–75 yr (DREW): Design and rationale. Med Sci Sports Exerc 2004;36:336-344. [Crossref] [PubMed]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 7th ed. Baltimore: Lippincott Williams & Wilkins, 2006.
- U.S. Department of Health and Human Services. Surgeon General’s report: Physical activity and health. Washington, DC: U.S. Department of Health and Human Services, 1996.
- Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 2009;43:1-2. [PubMed]
- HOWDEN EJ. Effects of excise and lifestyle intervention on cardiovascular function in CKD Clin J Am Soc Nephr Cjasn. 2013;8:1494-1501. [J]. [Crossref]
- Zhao W T, Liu X, Pang J Q, et al. Effects of FATmax Exercise Oil Physique and Cardiovascular Function in the Obese Elderly. China Sport Science. 2016;36:48-52. [J].
- Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723-7. [Crossref] [PubMed]
- Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond) 2011;35:427-35. [Crossref] [PubMed]
- Pierce GL, Eskurza I, Walker AE, et al. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2011;120:13-23. [Crossref] [PubMed]
- Rodrigo PS, Alemán JA, Jara PG, et al. Efectos de un programa de ejercicio de fuerza/resistencia sobre los factores de riesgo cardiovascular en mujeres posmenopáusicas de bajo riesgo cardiovascular. Estudio CLIDERICA. Aten Primaria 2008;40:351-6. [Crossref]
- Malandish A, Tartibian B, Sheikhlou Z, et al. The effects of short-term moderate intensity aerobic exercise and long-term detraining on electrocardiogram indices and cardiac biomarkers in postmenopausal women. J Electrocardiol 2020;60:15-22. [Crossref] [PubMed]
- Gómez-Tomás C, Chulvi-Medrano I, Carrasco JJ, et al. Effect of a 1-year elastic band resistance exercise program on cardiovascular risk profile in postmenopausal women. Menopause 2018;25:1004-10. [Crossref] [PubMed]
- Wong A, Figueroa A. The Effects of Low Intensity Resistance Exercise on Cardiac Autonomic Function and Muscle Strength in Obese Postmenopausal Women. J Aging Phys Act 2019;27:855-60. [Crossref] [PubMed]
- Dalleck LC, Allen BA, Hanson BA, et al. Dose-response relationship between moderate-intensity exercise duration and coronary heart disease risk factors in postmenopausal women. J Womens Health (Larchmt) 2009;18:105-13. [Crossref] [PubMed]
- Gerage AM, Forjaz CL, Nascimento MA, et al. Cardiovascular adaptations to resistance training in elderly postmenopausal women. Int J Sports Med 2013;34:806-13. [Crossref] [PubMed]
- Pekas EJ, Shin J, Son WM, et al. Habitual Combined Exercise Protects against Age-Associated Decline in Vascular Function and Lipid Profiles in Elderly Postmenopausal Women. Int J Environ Res Public Health 2020;17:3893. [Crossref] [PubMed]
- Jeon K, Lee S, Hwang MH. Effect of combined circuit exercise on arterial stiffness in hypertensive postmenopausal women: a local public health center-based pilot study. Menopause 2018;25:1442-7. [Crossref] [PubMed]
- Son WM, Park JJ. Resistance Band Exercise Training Prevents the Progression of Metabolic Syndrome in Obese Postmenopausal Women. J Sports Sci Med 2021;20:291-9. [Crossref] [PubMed]
- Lee JA, Kim JW, Kim DY. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause 2012;19:296-301. Erratum in: Menopause 2012;19:486. [Crossref] [PubMed]
- Nishiwaki M, Kawakami R, Saito K, et al. Vascular adaptations to hypobaric hypoxic training in postmenopausal women. J Physiol Sci 2011;61:83-91. [Crossref] [PubMed]
- Shen TW, Wen HJ. Aerobic exercise affects T-wave alternans and heart rate variability in postmenopausal women. Int J Sports Med 2013;34:1099-105. [Crossref] [PubMed]
- Son WM, Sung KD, Cho JM, et al. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause 2017;24:262-8. [Crossref] [PubMed]
- Zhang D, Janjgava T, Boutcher SH, et al. Cardiovascular response of postmenopausal women to 8 weeks of sprint interval training. Eur J Appl Physiol 2019;119:981-9. [Crossref] [PubMed]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [Crossref] [PubMed]
- Martins D, Nelson K, Pan D, et al. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med 2001;4:10-3, 20. [PubMed]
- Maslow AL, Sui X, Colabianchi N, et al. Muscular strength and incident hypertension in normotensive and prehypertensive men. Med Sci Sports Exerc 2010;42:288-95. [Crossref] [PubMed]
- LaStayo PC, Ewy GA, Pierotti DD, et al. The positive effects of negative work: increased muscle strength and decreased fall risk in a frail elderly population. J Gerontol A Biol Sci Med Sci 2003;58:M419-24. [Crossref] [PubMed]
- Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004;292:1188-94. [Crossref] [PubMed]
- Lee Y, Siddiqui WJ. Cholesterol Levels. Treasure Island, FL, USA: StatPearls Publishing LLC, 2019.
- Rosano GM, Vitale C, Marazzi G, et al. Menopause and cardiovascular disease: the evidence. Climacteric 2007;10:19-24. [Crossref] [PubMed]
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013;40:195-211. [Crossref] [PubMed]
- Wooten JS, Phillips MD, Mitchell JB, et al. Resistance exercise and lipoproteins in postmenopausal women. Int J Sports Med 2011;32:7-13. [Crossref] [PubMed]
- Ammar T. Effects of aerobic exercise on blood pressure and lipids in overweight hypertensive postmenopausal women. J Exerc Rehabil 2015;11:145-50. [Crossref] [PubMed]
- Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005;23:1839-46. [Crossref] [PubMed]
- Racette SB, Evans EM, Weiss EP, et al. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care 2006;29:673-8. [Crossref] [PubMed]
- Buemann B, Sørensen TI, Pedersen O, et al. Lower-body fat mass as an independent marker of insulin sensitivity--the role of adiponectin. Int J Obes (Lond) 2005;29:624-31. [Crossref] [PubMed]
- Conceição MS, Bonganha V, Vechin FC, et al. Sixteen weeks of resistance training can decrease the risk of metabolic syndrome in healthy postmenopausal women. Clin Interv Aging 2013;8:1221-8. [Crossref] [PubMed]
(English Language Editor: N. Korszniak)





