
The safety and effectiveness of α-synuclein immunotherapy vs. placebo for the treatment of Parkinson’s disease: a systematic review and meta-analysis
Highlight box
Key findings
• No statistical difference in the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale total score, adverse event incidence, headache incidence and constipation incidence.
What is known and what is new?
• Using α-synuclein as primary immunotherapy sites for Parkinson’s disease remains highly contentious.
What is the implication, and what should change now?
• There was no statistically significant difference in the AE incidence and MDS-UPDRS total score between the immunotherapy and control groups. Given the small number and varying quality of the included studies, high-quality, multi-center, and large-scale clinical studies are desired to corroborate our findings.
Introduction
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder that primarily affects older people (1). The prevalence of PD rises exponentially with age, reaching 1–2% in adults aged over 65 and 3–5% in those over 85 years old (2-4). It is mainly caused by the death of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the buildup of Lewy bodies, which led to a considerable drop in striatal content (5,6). PD features an insidious onset and slow progress. The main clinical manifestations include bradykinesia, myotonia, static tremor, and impaired postural reflexes. Meanwhile, PD patients may also be plagued by insomnia, depressive disorders, astriction, and other non-motor symptoms (7-9). Both motor and non-motor PD symptoms increasingly worsen as the illness progresses. Motor complications are frequent in the later stages of disease development, including declining drug efficacy, the “on-off” phenomenon, and dyskinesia (10). In the later stages, patients often cannot take care of themselves and may require long-term bed rest, with a poorer quality of life due to equilibrium disorders, falls, freezing of gait, deglutition disorders, and aphasia (11). Currently, the primary treatment of PD is drug therapy, and levodopa preparations are the most effective (12). Surgical treatment is merely an effective supplement to drug therapy; rehabilitation and psychotherapy can also improve symptoms to a certain extent. Nevertheless, none of these treatments slows or cures PD. Fortunately, recent studies have shown that immunotherapy has a grander prospect for the treatment of PD (13,14).
Immunotherapy in the treatment of PD predominantly involves the passive immune treatment of α-synuclein (α-syn) antibodies. PD’s primary pathological change is the buildup of Lewy bodies, which mainly comprise abnormally accumulated α-syn in the SNpc (15,16). Immunotherapy aims to eliminate the abnormally aggregated α-syn, which is widely considered the core of PD’s pathogenesis (17,18). Although there are more and more clinical studies on PD immunotherapy, Lang et al. (19) found no difference between PD immunotherapy and placebo, but Jankovic et al. (20) thought PD immunotherapy was safe and tolerable. Meta-analysis was able to synthesize relevant studies on immunotherapy for PD to obtain the latest results of immunotherapy and placebo for PD. Therefore, this study aims to synthesize the latest clinical evidence and provide new treatment options for PD patients. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1356/rc).
Methods
Search strategy
We searched the databases of CNKI, CBM, Cochrane Library, PubMed, Web of Science, and Embase for randomized controlled trials (RCTs) on immunization treatments for PD. The retrieval was from the establishment of the databases to September 1, 2022. Both medical subject headings (MeSH) and free words were searched, including PD, paralysis agitans, alpha-synuclein, antibody, and immunotherapy. No restrictions were imposed on regions or publication status.
Inclusion and exclusion criteria
The inclusion criteria were as follows: PD patients older than 18 years; the experimental group adopted immunotherapy, whereas the control group used placebos; the primary outcome was the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) total score and the secondary outcome was adverse reactions (headache, constipation).
The exclusion criteria were as follows: conference abstracts, duplicate articles, systematic reviews, meta-analyses, animal experiments, and case reports; unreasonable experimental design; studies with full text or data unavailable.
Data extraction
Two researchers screened the literature independently. Based on titles and abstracts, irrelevant studies were removed. The full texts of the remaining articles were downloaded and reviewed before 6 trials were found to be eligible for the present study. In the case of disagreement, relevant teachers were consulted for advice. After the literature screening, the 2 researchers collected observational data from the 6 eligible studies independently. Upon the completion of data extraction, the data results were cross-checked to ensure consistency. Extracted data included the year of publication, first author, gender, follow-up duration, country, sample size, intervention, age, and outcomes.
Quality assessment
Two investigators independently assessed the quality of the 6 eligible studies using the Cochrane Collaboration’s bias assessment tool (21). The quality evaluation involved 7 aspects: random sequence generation (selection bias), data integrity (attrition bias), selective reporting of research results (reporting bias), allocation concealment (selection bias), masked participants and implementers (performance bias), masked outcome assessors (observation bias), and other bias. Each study’s quality was evaluated based on the assessment standard mentioned above. If an original study completely met the standard, it was considered low risk, indicating relatively high quality. If an original study partially met the standard, it was rated as unclear risk, suggesting a moderate quality. If an original study did not meet the standard, it was rated as high risk, indicating low quality.
Statistical analysis
The software Stata 15.0 (Stata Corp., College Station, TX, USA) was employed for the statistical analysis. Additionally, continuous variables were reported as weighted mean difference (WMD) with 95% confidence intervals (CIs), and binary variables were expressed as relative risk (RR) with 95% CI. For each trial, a heterogeneity test was run. A P value ≥0.1 and I2<50% indicated small heterogeneity, and hence a fixed-effects model was employed for data analysis. In contrast, heterogeneity was indicated by P <0.1 and I2>50%. To investigate the cause of the heterogeneity, subgroup analyses were performed. If the cause of the heterogeneity could not be identified, a random-effects model was applied. Publication bias was examined through visual inspection of an egger test. If the publication bias is large, it will lead to poor credibility of our conclusions. A P<0.05 is two-sided suggested that the difference was statistically significant.
Results
Study selection process and results
The aforementioned databases initially yielded 3,168 articles. After deleting the repetitive literature, we identified 2,383 papers. Titles and abstracts were checked before 83 articles were screened out. Finally, a total of 6 RCTs were included after a full-text review (19,20,22-25). Figure 1 depicts the literature screening process.
Characteristics of the included studies
A total of 6 RCTs were eligible for the current study, with 606 immunotherapy recipients and 254 control individuals. Immunosuppressive agents comprised PRX002, cinpanemab, prasinezumab, and UB-312. The basic characteristics of the included studies are presented in Table 1.
Table 1
| Study | Country | Sample size | Gender (M/F) | Mean age (years) | Intervention | Follow-up (week) | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | ||||||
| Jankovic 2018 (20) | USA | 55 | 25 | 64/16 | 58.0 | 58.0 | PRX002 (0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg, 30 mg/kg, 60 mg/kg) | Placebo | 6 | F1 | |
| Lang 2022 (19) | USA | 262 | 100 | 250/127 | 60.1 | 61.0 | C (250 mg, 1,250 mg, 3,000 mg) | Placebo | 52 | F1; F2 | |
| Meissner 2020 (24) | France | 24 | 6 | 19/11 | PD01A:62; PD03A:60 | 63 | PD01A:0.75 mg; PD03A:0.75 mg | Placebo | 52 | F1 | |
| Pagano 2022 (23) | Switzerland | 211 | 105 | 213/103 | P1500 mg: 60.3; P4500 mg: 59.4 | 59.9 | P1500 mg; P4500 mg | Placebo | 52 | F1; F2 | |
| Schenk 2017 (25) | USA | 30 | 10 | 15/25 | 32.5–37 | 45.0 | PRX002 0.3 mg/kg; PRX002 1 mg/kg; PRX002 3 mg/kg; PRX002 10 mg/kg; PRX002 30 mg/kg | Placebo | 12 | F1 | |
| Yu 2022 (22) | USA | 24 | 8 | 15/17 | 60–72 | 70 | UB-312, 0.1 mg; UB-312, 0.3 mg; UB-312, 1 mg; UB-312, 3 mg | Placebo | 50 | F1 | |
EG, experimental group; CG, control group; C, cinpanemab; P, prasinezumab; F1, adverse event; F2; MDS-UPDRS total score; MDS-UPDRS, the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale.
Risk of bias assessment
All of the 6 eligible studies elucidated the specific methods for generating random sequences, although 1 of them did not explain its blinding method in detail. The 6 eligible studies were all of reasonably good quality. Figure 2 depicts the risk of bias graph, and Figure 3 shows the risk of bias summary.
Meta-analysis
Adverse events (AEs)
All 6 included studies described the adverse reaction outcome, with 606 immunotherapy recipients and 254 control individuals. Participants were divided into 3 subgroups by specific AEs: any adverse, treatment-related AEs, and serious AEs. A fixed-effects model was employed for data analysis based on the result of the heterogeneity test (I2=0%, P=0.785). No significant difference was noted in the AE incidence (RR: 1.06, 95% CI: 0.98 to 1.15, P=0.150). Specifically, no significant difference was observed in the incidence of any AE (I2=0%, P=0.375) (RR: 1.03, 95% CI: 0.96 to 1.11, P=0.393), the incidence of treatment-related AEs (I2=0%, P=0.521) (RR: 1.11, 95% CI: 0.85 to 1.45, P=0.460), and the incidence of serious AE (I2=0%, P=0.678) (RR: 1.31, 95% CI: 0.76 to 2.27, P=0.330). The meta-analysis of AEs is shown in Figure 4.
Headache
There were 5 studies (19,20,22,24,25) that reported the headache outcome, with 595 immunotherapy recipients and 248 control individuals. A fixed-effects model was employed for data analysis based on the heterogeneity test result (I2=0%, P=0.889). As shown in Figure 5, no significant difference was seen in headache incidence between the 2 groups (RR: 0.95, 95% CI: 0.67 to 1.34, P=0.773).
Infection
A total of 5 studies reported the infection outcome, including 595 immunotherapy recipients and 248 control individuals. A fixed-effects model was adopted for data analysis according to the heterogeneity test result (I2=4.2%, P=0.383). The infection rate in the immunotherapy group was greater than that in the control group (RR: 2.29, 95% CI: 1.40 to 3.74, P=0.003). The meta-analysis of infection is shown in Figure 6.
Constipation
A total of 3 studies (19,20,24) reported the constipation outcome, with 523 immunotherapy recipients and 230 control individuals. A fixed-effects model was used for data analysis based on the heterogeneity test result (I2=0%, P=0.584). No significant difference was observed in the incidence of constipation (RR: 1.47, 95% CI: 0.77 to 2.78, P=0.242). The meta-analysis of constipation is shown in Figure 7.
MDS-UPDRS total score
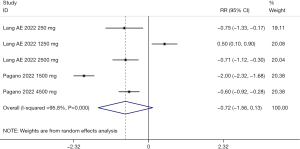
The MDS-UPDRS total score was reported in 2 studies (19,20), with 523 immunotherapy recipients and 230 control individuals. According to the heterogeneity test (I2=95.8%, P<0.001), a random-effects model was employed for data analysis. No statistical difference was seen in the MDS-UPDRS total score (WMD: −0.72, 95% CI: −1.56 to 0.13, P=0.099). The meta-analysis of MDS-UPDRS total score is shown in Figure 8. Due to high heterogeneity, we conducted a sensitivity analysis, in which the included studies were excluded successively. The analysis revealed a small sensibility, which suggested that our meta-analysis results were robust. The sensitivity analysis of the MDS-UPDRS total score is shown in Figure 9.

Publication bias
Egger test for the MDS-UPDRS total score and AEs were drawn to assess the publication bias. The P values of the two Egger tests were all greater than 0.05, indicating that there was no publication bias in the total MDS-UPDRS (P=0.392) and AEs (P=0.875). The funnel plot of the MDS-UPDRS total score is presented in Figure 10, and the funnel plot of AEs is shown in Figure 11.

Discussion
A fundamental pathological change of PD is the aberrant buildup of α-syn at the presynaptic terminals in a diseased state (26,27). The release of neurotransmitters is highly associated with soluble attachment proteins. This kind of protein can identify and bind to the v-SNARE receptor on vesicle membranes and the t-SNARE receptor on target films at vesicle docking sites, activating the assembly of the fusion complexes. The membrane fusion complex catalyzes the fusion of vesicles and target membranes. The α-syn can adjust this neurotransmitter release process. Therefore, α-syn is closely correlated with the release of neurotransmitters and the reabsorption of synaptic vesicles (28,29). According to another study, α-syn-related neurodegenerative diseases are often accompanied by inflammatory responses, suggesting that α-syn is essential in non-neuronal cells and the immune system (30). Sardi et al. (31) proposed that α-syn can serve as immunotherapeutic targets to alleviate α-syn’s abnormal accumulation in the extracellular matrix and α-syn’s diffusion in the brain.
A total of 6 RCTs were eligible for the present meta-analysis. No statistical difference was seen in the MDS-UPDRS total score (WMD: −0.72, 95% CI: −1.56 to 0.13, P=0.099). The evaluation of PD mainly relies on clinical manifestations, and there is no reliable, objective indicator. The MDS-UPDRS assessment tool is crucial for each center to evaluate the condition of PD patients, drug effects, and mutual exchanges (32). Since there are few studies reporting the MDS-UPDRS total score, the results should be interpreted with care. Nonetheless, we believe that α-syn immunotherapy for the treatment of PD will have great prospects with the increase in the number of related studies. Furthermore, no statistical difference was found in the AE incidence (RR: 1.06, 95% CI: 0.98 to 1.15, P=0.150), headache incidence (RR: 0.95, 95% CI: 0.67 to 1.34, P=0.773), and constipation incidence (RR: 1.47, 95% CI: 0.77 to 2.78, P=0.242). However, the infection rate in the immunotherapy group was higher than in the control group (RR: 2.29, 95% CI: 1.40 to 3.74, P=0.003). As a result, close attention should be paid to infection responses during the course of immunological therapy. Although the adverse reactions of immunotherapy were the same as those of placebos, all their adverse reactions were mild, at grade 2–3, with no influence on the immunotherapy.
The present study still has some limitations. Firstly, non-English databases were not searched and it included few studies and participants, so our results should be interpreted with care. Secondly, the different types and concentrations of drugs in the included studies may result in clinical application limitations. Thirdly, there was high heterogeneity across the included studies, but the subgroup analysis failed to identify the cause of heterogeneity due to limited data in the original studies.
Conclusions
Existing results showed that α-syn immunotherapy had no significant effect on PD. Given the small number and varying quality of the included studies, high-quality, multi-center, and large-scale clinical studies are desired to corroborate our findings.
Acknowledgments
The authors thank all researchers and participants for sharing these data.
Funding: The work was funded by Lianyungang sixth 521 high-level talent training project (No. LYG06521202104)
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1356/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1356/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Homayoun H. Parkinson Disease. Ann Intern Med 2018;169:ITC33-48. [Crossref] [PubMed]
- Mullard A. Parkinson disease setback. Nat Rev Drug Discov 2020;19:373. [PubMed]
- Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020;323:548-60. [Crossref] [PubMed]
- Maetzler W, Berg D. Parkinson disease in 2017: Changing views after 200 years of Parkinson disease. Nat Rev Neurol 2018;14:70-2. [Crossref] [PubMed]
- Bae YJ, Kim JM, Sohn CH, et al. Imaging the Substantia Nigra in Parkinson Disease and Other Parkinsonian Syndromes. Radiology 2021;300:260-78. [Crossref] [PubMed]
- Vitali P, Pan MI, Palesi F, et al. Substantia Nigra Volumetry with 3-T MRI in De Novo and Advanced Parkinson Disease. Radiology 2020;296:401-10. [Crossref] [PubMed]
- Savica R, Boeve BF, Mielke MM. When Do α-Synucleinopathies Start? An Epidemiological Timeline: A Review. JAMA Neurol 2018;75:503-9. [Crossref] [PubMed]
- Lewis SJ. Disease-modifying approaches for Parkinson disease. Med J Aust 2018;208:377-8. [Crossref] [PubMed]
- Chew EG, Foo JN, Tan EK. Identifying genes in Parkinson disease: state of the art. Med J Aust 2018;208:381-2. [Crossref] [PubMed]
- Chen-Plotkin AS. Parkinson disease: Blood transcriptomics for Parkinson disease? Nat Rev Neurol 2018;14:5-6. [Crossref] [PubMed]
- Malkki H. Parkinson disease: Could gut microbiota influence severity of Parkinson disease? Nat Rev Neurol 2017;13:66-7. [Crossref] [PubMed]
- Aradi SD, Hauser RA. Medical Management and Prevention of Motor Complications in Parkinson's Disease. Neurotherapeutics 2020;17:1339-65. [Crossref] [PubMed]
- Fyfe I. Parkinson disease: T cells recognize α-synuclein peptides in Parkinson disease. Nat Rev Neurol 2017;13:450-1. [Crossref] [PubMed]
- Chaudhuri KR, Sauerbier A. Parkinson disease. Unravelling the nonmotor mysteries of Parkinson disease. Nat Rev Neurol 2016;12:10-1. [Crossref] [PubMed]
- Jamal F. Immunotherapies Targeting α-Synuclein in Parkinson Disease. Fed Pract 2020;37:375-9. [PubMed]
- Antonini A, Bravi D, Sandre M, et al. Immunization therapies for Parkinson's disease: state of the art and considerations for future clinical trials. Expert Opin Investig Drugs 2020;29:685-95. [Crossref] [PubMed]
- Tiwari RK, Moin A, Rizvi SMD, et al. Modulating neuroinflammation in neurodegeneration-related dementia: can microglial toll-like receptors pull the plug? Metab Brain Dis 2021;36:829-47. [Crossref] [PubMed]
- Hadi F, Akrami H, Totonchi M, et al. α-synuclein abnormalities trigger focal tau pathology, spreading to various brain areas in Parkinson disease. J Neurochem 2021;157:727-51. [Crossref] [PubMed]
- Lang AE, Siderowf AD, Macklin EA, et al. Trial of Cinpanemab in Early Parkinson’s Disease. N Engl J Med 2022;387:408-20. [Crossref] [PubMed]
- Jankovic J, Goodman I, Safirstein B, et al. Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol 2018;75:1206-14. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Yu HJ, Thijssen E, van Brummelen E, et al. A Randomized First-in-Human Study With UB-312, a UBITh® α-Synuclein Peptide Vaccine. Mov Disord 2022;37:1416-24. [Crossref] [PubMed]
- Pagano G, Taylor KI, Anzures-Cabrera J, et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N Engl J Med 2022;387:421-32. [Crossref] [PubMed]
- Meissner WG, Traon AP, Foubert-Samier A, et al. A Phase 1 Randomized Trial of Specific Active α-Synuclein Immunotherapies PD01A and PD03A in Multiple System Atrophy. Mov Disord 2020;35:1957-65. [Crossref] [PubMed]
- Schenk DB, Koller M, Ness DK, et al. First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 2017;32:211-8. [Crossref] [PubMed]
- Zhang H, Liu X, Liu Y, et al. Crosstalk between regulatory non-coding RNAs and oxidative stress in Parkinson’s disease. Front Aging Neurosci 2022;14:975248. [Crossref] [PubMed]
- Zago E, Dal Molin A, Dimitri GM, et al. Early downregulation of hsa-miR-144-3p in serum from drug-naïve Parkinson’s disease patients. Sci Rep 2022;12:1330. [Crossref] [PubMed]
- Xie L, Hu L. Research progress in the early diagnosis of Parkinson’s disease. Neurol Sci 2022;43:6225-31. [Crossref] [PubMed]
- Vardanyan R, König HH, Hajek A. Association between Parkinson’s Disease and Psychosocial Factors: Results of the Nationally Representative German Ageing Survey. J Clin Med 2022;11:4569. [Crossref] [PubMed]
- Rosenblum S, Meyer S, Richardson A, et al. Capturing Subjective Mild Cognitive Decline in Parkinson’s Disease. Brain Sci 2022;12:741. [Crossref] [PubMed]
- Sardi SP, Cedarbaum JM, Brundin P. Targeted Therapies for Parkinson’s Disease: From Genetics to the Clinic. Mov Disord 2018;33:684-96. [Crossref] [PubMed]
- Castro Caldas A, Correia Guedes L, Ferreira JJ, et al. Musician’s Dystonia as the Initial Presentation of Parkinson’s Disease. Mov Disord Clin Pract 2016;3:624-5. [Crossref] [PubMed]
(English Language Editor: J. Jones)










