
Systemic immunoglobulin light chain (AL) amyloidosis initially presenting as hematochezia: a case report
Introduction
Amyloidosis is an umbrella term encompassing a large group of disorders featuring the misfolding of soluble precursor proteins, ultimately generating highly ordered amyloid cross β-fibril molecules that deposit in multiple tissues (1). While multiple proteins can form amyloid fibrils, immunoglobulin light chain, transthyretin, serum amyloid A, and Aβ2 amyloid are most frequently involved (2). Here, we present a case of systemic immunoglobulin light chain (AL) amyloidosis with colonic and renal involvement.
Colonic amyloidosis commonly shows nonspecific signs. Physicians should consider the possibility of amyloidosis in patients complaining of hematochezia who also have various symptoms involving multiple organ systems. Amyloidosis should be considered even if the functional impairment of each organ has a corresponding previous history, as was the case for the patient described here. Gastroenterologists should consider underlying AL amyloidosis after detecting submucosal hematomas of the gastrointestinal (GI) tract during endoscopic examination. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-740/rc).
Case presentation
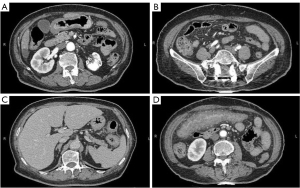
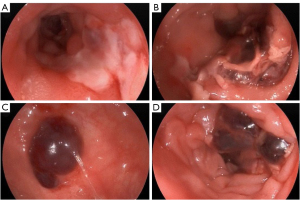
A 75-year-old woman presented to Beijing Friendship Hospital with intermittent lower abdominal pain, hematochezia, and a weight loss of 10 kg in the past year. Every 2–3 months, she experienced roughly the same attack, which presented as hypogastralgia accompanied by 8 to 10 moderate bloody stools per day. Abdominal computed tomography (CT) showed extensive thickening of the colon wall. Thickening and exudation of the adjacent omentum and mesentery were detected with a few peripheral lymph nodes (Figure 1). Gastroscopy showed reflux esophagitis (Los Angeles grade A) (LA-A). A colonoscopy revealed multiple discrete ulcers and submucosal hematomas of various sizes from the transverse colon to the sigmoid colon. The ulcers were circular or longitudinal with clear boundaries (Figure 2). A biopsy showed nonspecific chronic inflammation, exudation, and granulation tissue. The patient’s abdominal pain and hematochezia were relieved through bowel rest and total parental nutrition for 1–2 weeks.
The patient had many comorbidities, including left renal tuberculosis (TB) and hilar lymph node TB for 59 years, type 2 diabetes for 8 years, and chronic kidney disease for 3 years with serum creatinine fluctuating around 120 µmol/L. She had coronary heart disease (CHD) and paroxysmal atrial fibrillation for the previous 3 months but had not received anticoagulation therapy. She had also experienced numbness in her toe tips in recent months. She denied being exposed to sick individuals or insect bites and had no recent travel history.
At admission, her body temperature was 36.1 ℃, and her blood pressure was 96/47 mmHg. On physical examination, she exhibited overt anemia. Lower abdomen tenderness was present, with no rebound pain or muscle tension. The remainder of the physical examination was unremarkable. Initial laboratory investigations are shown in Table 1. Electrocardiography (ECG) showed low voltage in the limb leads. The patient’s cardiac function was normal on the echocardiogram. A mesenteric artery ultrasound revealed no obvious stenosis. Based on the above examination results, no obvious evidence of active infection, drug-related colitis, lymphoma, ischemic colitis, intestinal tuberculosis, or inflammatory bowel disease was found.
Table 1
| Laboratory test | Results [normal range] |
|---|---|
| Serum | |
| HGB, g/L | 74 [115–150] |
| ESR, mm/1 hour | 125 [0–20] |
| Scr, μmol/L | 166.3 [41–111] |
| Serum albumin, g/L | 27.3 [40–55] |
| NT-proBNP, ng/L | 6340 [0–900] |
| Interferon gamma release assays, SFCs/106 PBMC | A >120 [0–24]; B 100 [0–24] |
| WBC, PLT, coagulation function, PCT, myocardial enzymes, autoantibody series, and CEA | Normal |
| CMV-DNA, EBV-DNA | Negative |
| Serum immunoglobulin, mg/dL | IgG 1,840 [700–1,600] |
| IgM 22.6 [40–230] | |
| IgA 46.6 [70–400] | |
| Urine | |
| Urine routine | Protein 2+ (negative) |
| UTP, mg/24 h | 2,097.48 [28–141] |
| Stool | |
| Smear and culture | Negative |
| Toxin A and B for Clostridium difficile | Negative |
| Fecal calprotectin | >1,800 μg/g |
HGB, hemoglobin; ESR, erythrocyte sedimentation rate; Scr, serum creatinine; NT-proBNP, N-terminal pro-B–type natriuretic peptide; SFC, spot forming cell; PBMC, peripheral blood mononuclear cell; WBC, white blood cell; PLT, platelet; PCT, procalcitonin; CEA, carcinoembryonic antigen; CMV-DNA, cytomegalovirus-deoxyribonucleic acid; EBV-DNA, Epstein-Barr virus DNA; UTP, urinary total protein; IgG, immunoglobulin G; IgM, immunoglobulin M; IgA, immunoglobulin A.
The patient subsequently underwent repeat colonoscopy, which showed multiple discrete ulcers that extended from the ascending to the descending colon. Larger ulcers in the ascending and transverse colon involved more than half of the lumen (Figure 3). Biopsy from the margin of the ascending colon ulcer revealed an extensive deposit of pink amorphous material in granulation tissue, which stained positive for Congo red and showed apple-green birefringence in polarized light (Figure 4). The pathology presented no evidence of cytomegalovirus (CMV), Epstein-Barr virus (EBV), or tuberculosis infection.

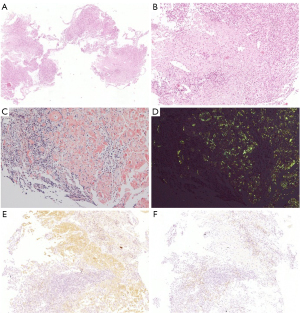
Primary or reactive amyloidosis was further differentiated. Proteinuria electrophoresis showed the presence of Bence-Jones proteins. Serum immunofixation electrophoresis revealed immunoglobin G (IgG)-κ type M proteinemia with elevated free κ light chain levels (5.29 g/L; normal range 1.7–3.7 g/L) and decreased free λ light chain levels (0.73 g/L; normal range 0.9–2.1 g/L). Immunohistochemistry of the colon biopsy revealed that the deposits were positive and negative for κ and λ light chains, respectively, indicating κ-light chain restriction (Figure 4). Bone marrow aspiration and biopsy analyses demonstrated a rate of 2.5% for atypical monoclonal plasma cells with positive κ staining consistent with plasma cell dyscrasia. Based on the above findings, a diagnosis of systemic AL amyloidosis with colon and kidney involvement was established.
The patient was transferred to the hematology ward for treatment. She was administered only bortezomib and dexamethasone for 4 cycles with no significant adverse reaction. At the follow-up, free κ light chain levels fluctuated, but the overall trend was downward. Her urinary protein and creatinine levels remained stable. Echocardiography was performed at 6- to 12-month intervals with no signs of cardiac amyloidosis. The numbness of her toe tips was alleviated. The severity and frequency of lower abdominal pain and hematochezia decreased. A follow-up colonoscopy 2 years after she initially presented to the hospital showed multiple, discrete shallow ulcerations distributed from the descending colon to the rectum with persistent amyloid deposition on biopsy. Due to her age, organ function, and family’s economic circumstances, the patient did not receive further stem cell transplants or additional chemotherapy regimens. As of writing, she is still being followed-up.
This case report has been approved by the Bioethics Committee of Beijing Friendship Hospital, Capital Medical University (No. 2022-P2-068-01). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Systemic AL amyloidosis is a malignant disease with monoclonal Ig light chains, λ, or less frequently κ, produced by a few bone marrow plasma cell clones. These monoclonal Ig light chains accumulate extracellularly as misfolded, insoluble protein complexes, which are referred to as amyloids (2). This disorder represents the most common type of amyloidosis, and the estimated yearly incidence is 10 to 12 cases per million individuals. It is a disease of older age, with a median patient age of 63 years (1).
The symptoms of amyloidosis observed in the clinic are associated with the affected organs. Amyloid accumulation in the heart generally manifests as restrictive cardiomyopathy, whereas cases with renal involvement present with proteinuria or impaired kidney function. Distal sensory impairment or motor neuropathy resulting from peripheral neuropathy can also occur (3). GI amyloid light chain amyloidosis manifests as fatigue, anorexia, abdominal pain, weight loss, GI bleeding, malabsorption, protein-losing gastroenteropathy, motility diseases, and liver enlargement (4). GI bleeding is detected in 4% to 36% of digestive tract amyloidosis cases and may result from erosions, ulcerations, or generalized oozing with no precise cause (2). Multiple mechanisms have been proposed to explain how amyloidosis induces intestinal hemorrhage. A potential explanation is that AL amyloidosis is associated with coagulation disorders (5). Another potential reason is that amyloid deposits intruding on the perivascular and vascular walls make these walls fragile, impairing vasoconstriction and causing mucosal erosion and bleeding (6). Intestinal hemorrhage may stop spontaneously without intervention but tends to relapse, requiring endoscopic hemostasis or surgery (2).
Abdominal CT might reveal edematous wall thickening of the small intestine and colon (7), which was found in the imaging findings in the patient described in this case report. Endoscopic findings are nonspecific and include erosion, ulceration, stricture, easy mucosal bleeding, submucosal hematoma, mucosal atrophy, gastric thickening folds, and granular/polypoid protrusions (4). The submucosal hematoma detected in our patient’s colon is unique to AL amyloidosis (8). Underlying AL amyloidosis should be considered if submucosal hematomas of the gut are detected by endoscopy.
Clinical and endoscopic findings in amyloidosis may be similar to those of other GI tract disorders. In our patient , inflammatory bowel disease was initially suspected based on endoscopic findings. However, neither crypt abscess nor granuloma was found in any biopsied tissues from the colon. A past medical history of diabetes and CHD combined with the colonoscopy results suggested the possibility of ischemic colitis, but no evidence of mesenteric vascular stenosis was observed. Cancer and lymphoma were also excluded because the biopsy showed no malignant cells. This case was even more uncommon because of the patient’s history of TB, which led us to erroneously believe the colon lesion resulted from intestinal tuberculosis. However, negative fast-acid staining and tuberculosis polymerase chain reaction of the colon biopsy did not support the diagnosis.
The gold standard for amyloidosis diagnosis is taking a tissue biopsy from an affected organ that shows positivity in Congo red staining and green birefringence in polarized light (9). Defining the amyloid type is crucial for making an accurate diagnosis and initiating the appropriate therapy. The most efficient techniques for discerning the amyloid type are immunohistochemistry, immunoelectron microscopy, and mass spectrometry (1).
Upon confirmation of systemic amyloidosis, cases should be examined for bone marrow malignancy, cardiomyopathy, and nephropathy (9). The amounts of N-terminal pro-B–type natriuretic peptide (NT-proBNP) increase soon after cardiac involvement, and mild proteinuria might be the initial sign of kidney involvement (1). In the current patient, renal puncture was not performed because of possible coagulation disorders. Her moderate renal insufficiency and nephrotic-range proteinuria combined with the results of proteinuria electrophoresis suggested renal involvement. The low voltage on ECG and cardiac failure with a preserved ejection fraction suggested the possibility of cardiac involvement despite the patient’s history of CHD. The patient did not meet the criteria for multiple myeloma but had plasma cell dyscrasia with 2.5% atypical monoclonal plasma cells in her bone marrow. The evidence of the involvement of other organs, such as the liver and skin, in this patient was not obvious. However, the numbness of the patient’s toe tips could not exclude diabetic peripheral neuropathy or amyloid deposition in the peripheral nervous system.
Systemic amyloidosis usually has a poor prognosis because most cases are detected in the late disease phase with multi-organ dysfunction, restricting treatment interventions. To detect the disease early, comprehensive analysis is needed, including detailed medical history, physical examination, laboratory tests, imaging, and endoscopic and pathological studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-740/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-740/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-740/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This case report has been approved by Bioethics Committee of Beijing Friendship Hospital, Capital Medical University (No. 2022-P2-068-01). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fotiou D, Dimopoulos MA, Kastritis E, Systemic AL. Amyloidosis: Current Approaches to Diagnosis and Management. Hemasphere 2020;4:e454. [Crossref] [PubMed]
- Talar-Wojnarowska R, Jamroziak K. Intestinal amyloidosis: Clinical manifestations and diagnostic challenge. Adv Clin Exp Med 2021;30:563-70. [Crossref] [PubMed]
- Ussia A, Vaccari S, Lauro A, et al. Colonic Perforation as Initial Presentation of Amyloid Disease: Case Report and Literature Review. Dig Dis Sci 2020;65:391-8. [Crossref] [PubMed]
- Latorre G, Vargas JI, Espino A. Endoscopic features of gastrointestinal amyloid light-chain amyloidosis. Lancet Gastroenterol Hepatol 2021;6:874. [Crossref] [PubMed]
- Mumford AD, O'Donnell J, Gillmore JD, et al. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol 2000;110:454-60. [Crossref] [PubMed]
- Simms LN, Suarez LSK, Deeb K, et al. The 13-year bleed: Exuberant amyloid angiopathies, angiodysplasias, and acquired coagulopathies of the gut. SAGE Open Med Case Rep 2021;9:2050313X211040018.
- Kim YJ, Kim HS, Park SY, et al. Intestinal amyloidosis with intractable diarrhea and intestinal pseudo-obstruction. Korean J Gastroenterol 2012;60:172-6. [Crossref] [PubMed]
- Yoshii S, Mabe K, Nosho K, et al. Submucosal hematoma is a highly suggestive finding for amyloid light-chain amyloidosis: Two case reports. World J Gastrointest Endosc 2012;4:434-7. [Crossref] [PubMed]
- Rowe K, Pankow J, Nehme F, et al. Gastrointestinal Amyloidosis: Review of the Literature. Cureus 2017;9:e1228. [PubMed]


