
Lung infection caused by Blastoschizomyces capitatus without immunodeficiency: a case report and review of the literature
Highlight box
Key findings
• This case demonstrates that
What is known and what is new?
•
• The patient reported herein had no evidence of a primary immunodeficiency disorder. The cause of his lung infection was established by mass spectrometry and metagenomic next-generation sequencing as
What is the implication, and what should change now?
• For pulmonary infection with a history of environmental exposure, early pathogen culture and identification, early diagnosis and early combination anti-fungal treatment are the key to successful management.
Introduction
Blastoschizomyces capitatus, also known as Geotrichum capitatum, is a species of the genus Geotrichum, which exists in natural environments such as soil and human skin, and can invade the human body through the respiratory and digestive tracts. It is a rare opportunistic fungus with no established treatment protocol according to recent international guidelines (1). According to a review of previous literatures, Blastoschizomyces capitatus is more common in patients with hematological malignancy and those with immunodeficiencies after transplantation (2-6). There are rare reports of Blastoschizomyces capitatus infection in immunocompetent patients, and the clinical manifestations are broadly similar to other fungal infections. Usually, diagnosis is by blood culture, confirmed in some patients by sputum culture. The patient reported herein had no evidence of a primary immunodeficiency disorder. The cause of his lung infection was established by mass spectrometry (MS) and metagenomic next-generation sequencing (mNGS) as Blastoschizomyces capitatus. At present, there is no very effective treatment for Blastoschizomyces capitatus infection. Here we show that Amphotericin B combined with voriconazole can achieve curative effect. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1289/rc).
Case presentation
The patient was a 67-year-old man, a smoker (of 15 pack-years), and a farmer, who was admitted to our hospital on 17th August 2021 with cough, expectoration, and fever which had been present for 2 months. His symptoms had progressed and had been accompanied by wheezing over the previous 20 days. Two months before, the patient had noted intermittent cough and expectoration with no obvious predisposing cause. He produced large amounts of greyish-yellow phlegm, and was accompanied by fever. His body temperature fluctuated between 37.3 ℃ and 37.5 ℃. He was treated with oral amoxicillin and roxithromycin in a clinic, with no obvious benefit. Twenty days before admission, his body temperature had increased significantly, reaching 39.2 ℃, accompanied by cold intolerance, intermittent chills, and progressive cough with expectoration, and the development of wheezing. Enhanced computed tomography (CT) of the chest and abdomen showed evidence of old tuberculosis in the upper lobe of the right lung, multiple slightly enlarged lymph nodes in the mediastinum, subpleural pulmonary nodules in the lower lobe of the right lung, and inflammation in both lower lobes, with emphysema and bullae. However, there was no abnormality noted in the abdomen (Figure 1). He was admitted to the Department of Respiratory Medicine, The Fourth Hospital of Hebei Medical University, on 17th August 2021.
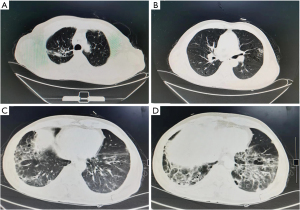
The patient had a history of pulmonary tuberculosis and tuberculous pleurisy 6 years before. One month prior to admission, he had cleaned a small bungalow, which had been unoccupied for a long time. He had adopted no protective measures during the cleaning process, and he mentioned that the bungalow had smelt distinctly of mold.
Pre-admission physical examination results included the following: body temperature: 38.8 ℃, pulse: 99 beats/min regular, respiration: 23 breaths/min, blood pressure: 128/78 mmHg, and oxygen saturation by pulse oximeter of 90%. He appeared acutely ill and was breathless at rest and tachypneic. No jaundice was observed on his skin or sclera, and no rash or palpable enlargement of superficial lymph nodes was present throughout the body. Both of the lungs were resonant on percussion; however, moist rales were heard in both lower lobes of the lungs. No abnormalities were found on physical examination of the heart or abdomen, but pitting edema was observed in both lower limbs. The diagnosis upon admission included the following: (I) lesions in both lungs. (II) Old pulmonary tuberculosis in the right upper lobe.
Following admission, the investigations showed the following: routine blood count: white blood cells: 14.51×109/L [normal range (NR) 3.5–9.5×109/L], neutrophils: 13.39×109/L (NR 1.8–6.3×109/L), lymphocytes: 0.76×109/L (NR 1.1–3.2×109/L), red blood cells: 3.56×1012/L (NR 4.3–5.8×1012/L), hemoglobin: 114 g/L (NR 130–175 g/L), platelets: 346×109/L (NR 125–350×109/L); coagulation function: fibrinogen: 6.94 g/L (NR 2.38–4.98 g/L), fibrinogen degradation products: 18.27 mg/L (NR <5 mg/L), D-dimer: 2.339 mg/L (NR <0.243 mg/L); prealbumin: 50.1 mg/L (NR 200–430 mg/L), albumin: 23.6 g/L (NR 40–55 g/L), other liver function tests were normal; renal function, cardiac enzymes, and troponin I were normal. Hepatitis A, B, and C antibodies; syphilis; and HIV serology were negative. Other blood results included the following: erythrocyte sedimentation rate (ESR): 92 mm/h (NR 0–15 mm/h), C-reactive protein (CRP): 170 mg/L (NR <10 mg/L), procalcitonin: 0.24 ng/mL (NR <0.5 ng/mL), G (glucan): 424.5 pg/mL (NR <60 pg/mL), and galactomannan (GM): 0.65 (positive, if ≥0.5). Sputum acid-fast stain was performed three times; purified protein derivative (PPD) testing and interferon-gamma release assay gave negative results as did antinuclear antibodies, anti-streptolysin O (ASO), rheumatoid factor (RF), and antineutrophil cytoplasmic antibody (ANCA). Echocardiography showed an ejection fraction of 65% and reduced left ventricular diastolic function. Ultrasound of the deep veins of both lower limbs showed no abnormality. Ultrasound of the urinary system showed prostatic enlargement. Bone marrow aspiration and biopsy showed toxic granulation and features of infection.
Following admission, the patient received nutritional support and intravenous piperacillin sodium 4.0 g and tazobactam sodium 0.5 g q8h (every 8 hours) as anti-infective treatment for 5 days. However, there was no general improvement, and his temperature did not drop. In order to identify the putative cause, basal lung infection sputum was sent for a culture on 3 separate occasions, and bronchoscopic bronchoalveolar lavage fluid (BALF) was sent for routine bacterial and fungal culture and mNGS and MS. Sabouraud dextrose agar (SDA) was used for sputum culture. Blastoschizomyces capitatus was isolated on SDA culture, with a positive smear (Figure 2). The BALF mNGS was consistent with Blastoschizomyces capitatus, Sequence 129.
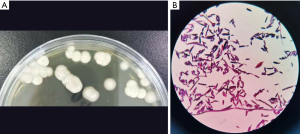
Following adjustment of the anti-microbial therapy to voriconazole, initial loading dose of 400 mg q12h (every 12 hours) followed by a maintenance dose of 200 mg, twice daily, in combination with amphotericin B 0.25% solution via nebulizer twice daily, the patient’s temperature normalized after 24 hours. After 5 days of treatment, the symptoms of cough and expectoration improved, and repeat serum G and GM tests both normalized at 49.6 pg/mL and 0.25, respectively. After 14 days of therapy, repeat sputum culture was negative. The medication was well tolerated with no hypokalemia and persistently normal renal function. Amphotericin B was discontinued, and the patient was discharged due to general clinical improvement, reduction in total white cells to 8.10×109/L and in neutrophils: to 5.81×109/L, and more than halving of CRP down to 76.8 pg/mL. A repeat CT scan also showed improvement in pulmonary inflammatory changes. Following discharge, he continued to take voriconazole orally for 2 weeks, and sputum culture remained negative at 1 month follow-up. Important clinical information and main treatment of the patient in a timeline (Figure 3). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Blastoschizomyces capitatus infection is a very rare fungal infection that occurs most often in patients who are immunocompromised, particularly in those with hematological malignancy, neutropenia, or who have undergone organ transplantation (2-6). Systemic infection caused by Blastoschizomyces capitatus may involve the lung, liver, kidney, bone marrow, central nervous system, heart, etc. (7-9) and case fatality rate is over 50% (10,11). Diagnosis of Blastoschizomyces capitatus can be confirmed by blood and sputum culture, and the positive rate of detection by blood culture may reach 70% (12).
In our patient, there was no history of primary immunodeficiency. The diagnosis was confirmed by MS of sputum culture and mNGS of BALF, and positive smear and sputum culture on SDA, although the blood culture was negative. The 1,3-β glucan serology test, which detects fungal cell wall components and may be useful in the early diagnosis of fungal infection, gave a positive G test result in our patient. Interestingly, the GM test, detecting galactomannan, which is mainly used for the early diagnosis of Aspergillus infection, was also positive. There was no evidence of Aspergillus infection in this patient, suggesting that Blastoschizomyces capitatus led to a positive GM test, as previously reported (12). Alternative diagnoses were considered: tuberculosis was excluded by 3 negative results of sputum, and BALF, acid-fast staining and culture, negative PPD test, and negative interferon-gamma release assay. Furthermore, no evidence of tubercle bacilli was found on mNGS of BALF. The possibility of autoimmune disease was excluded by the absence of any clinical features and negative antinuclear, RF, and ANCA antibodies.
Our case highlights the potential advantages of MS and mNGS methods in identifying fungal and rare bacterial infections as recently reviewed (13).
Due to Blastoschizomyces capitatus infection being quite rare, a clearly recommended treatment regimen has not yet been developed. Studies have suggested that monotherapy with amphotericin B or voriconazole had poor therapeutic effects (14,15). Conversely, voriconazole (16), posaconazole (17), isavuconazole monotherapy (18), and even fluconazole (in immunocompetent patients) have all been reported to be effective when used as a treatment (15), whereas amphotericin B monotherapy has also shown good therapeutic effects in some patients (19) but not in others even when combined with fluconazole or micafungin (2,20). Becchetti et al. reported treatment success in a liver transplant patient with liposomal amphotericin B (3). Etienne et al. reported successful treatment with combination therapy of voriconazole and caspofungin (21). Shan et al. reported that the combination therapy of amphotericin B liposomes and caspofungin improved the clinical picture (6). The literature suggests that combination therapy has a higher success rate than monotherapy.
Voriconazole is a second-generation, triazole, antifungal drug, which has broad-spectrum antifungal effects. Its mechanism of action is to inhibit the demethylation of 14α-sterol mediated by cytochrome P450 in fungi, thus inhibiting the biosynthesis of ergosterol. Amphotericin B is a polyene antifungal drug, and its mechanism of action involves binding with sterol on the cell membrane of sensitive fungi, damaging cell membrane permeability, destroying normal cellular metabolism and inhibiting growth. Aerosol inhalation of amphotericin B has the potential to reduce the adverse reactions of systemic medication. Our case suggests that combination therapy with voriconazole and nebulized amphotericin B may be a useful new regimen for the treatment of Blastoschizomyces capitatus infection. In summary, the incidence of Blastoschizomyces capitatus infection is low, the clinical symptoms are non-specific, and the diagnosis is difficult. According to the patient’s medical history and clinical manifestations, CT and other examinations, pathogenic NGS, combined with conventional GM ang G testing, culture identification, can greatly assist diagnosis. Early diagnosis and early combination anti-fungal treatment are the key to successful management.
Conclusions
Blastoschizomyces capitatus infection can occur without immunodeficiency. The MS and mNGS methods have potential advantages in diagnosing rare fungal and bacterial infections. Combination therapy with voriconazole and aerosolized amphotericin B can be used as a new regimen for the treatment of Blastoschizomyces capitatus infection.
Acknowledgments
The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1289/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bupha-Intr O, Butters C, Reynolds G, et al. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern Med J 2021;51:177-219. [Crossref] [PubMed]
- Ben Neji H, Bchir M, Hamdoun M, et al. Geotrichum capitatum fungemia in patients treated for acute leukemia. Med Mal Infect 2019;49:284-6. [Crossref] [PubMed]
- Becchetti C, Ferrarese A, Cattelan A, et al. Geotrichum capitatum Invasive Infection Early After Liver Transplant. Exp Clin Transplant 2020;18:737-40. [Crossref] [PubMed]
- Oya S, Muta T. Breakthrough infection of Geotrichum capitatum during empirical caspofungin therapy after umbilical cord blood transplantation. Int J Hematol 2018;108:558-63. [Crossref] [PubMed]
- Fouassier M, Joly D, Cambon M, et al. Geotrichum capitatum infection in a neutropenic patient. Apropos of a case and review of the literature. Rev Med Interne 1998;19:431-3. [Crossref] [PubMed]
- Shan W, Dai C, Kan J, et al. Fungal Infection Caused by Geotrichum capitatum in a Severe Aplastic Anemia Patient: a Case Report and Review of the Literature. Clin Lab 2018;64:867-9. [Crossref] [PubMed]
- Lafayette TC, Oliveira LT, Landell M, et al. Dipodascus capitatus (Geotrichum capitatum): fatal systemic infection on patient with acute myeloid leukemia. Rev Soc Bras Med Trop 2011;44:648-50. [Crossref] [PubMed]
- Pimentel JD, Baker M, Woodgyer AJ, et al. Fatal disseminated Blastoschizomyces capitatus (Geotrichum capitatum) in a patient with relapse of acute lymphoblastic leukaemia. Pathology 2005;37:319-21. [Crossref] [PubMed]
- Özkaya-Parlakay A, Cengiz AB, Karadağ-Öncel E, et al. Geotrichum capitatum septicemia in a hematological malignancy patient with positive galactomannan antigen: case report and review of the literature. Turk J Pediatr 2012;54:674-8. [PubMed]
- Girmenia C, Pagano L, Martino B, et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 2005;43:1818-28. [Crossref] [PubMed]
- Martino R, Salavert M, Parody R, et al. Blastoschizomyces capitatus infection in patients with leukemia: report of 26 cases. Clin Infect Dis 2004;38:335-41. [Crossref] [PubMed]
- Giacchino M, Chiapello N, Bezzio S, et al. Aspergillus galactomannan enzyme-linked immunosorbent assay cross-reactivity caused by invasive Geotrichum capitatum. J Clin Microbiol 2006;44:3432-4. [Crossref] [PubMed]
- Tsang CC, Teng JLL, Lau SKP, et al. Rapid Genomic Diagnosis of Fungal Infections in the Age of Next-Generation Sequencing. J Fungi (Basel) 2021;7:636. [Crossref] [PubMed]
- Saghrouni F, Abdeljelil JB, Youssef YB, et al. Geotrichum capitatum septicemia in patients with acute myeloid leukemia. Report of three cases. Med Mycol Case Rep 2012;1:88-90. [Crossref] [PubMed]
- Trabelsi H, Néji S, Gargouri L, et al. Geotrichum capitatum septicemia: case report and review of the literature. Mycopathologia 2015;179:465-9. [Crossref] [PubMed]
- Wu Y, Ye Y, Yang Y, et al. Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: a case report. BMC Infect Dis 2021;21:842. [Crossref] [PubMed]
- Brunetti G, Visconti V, Ghezzi MC, et al. Management and treatment of Magnusiomyces capitatus (Geotrichum capitatum) pleural infection in a non-neutropenic patient with posaconazole. A new therapeutic opportunity? New Microbiol 2016;39:307-9. [PubMed]
- Esposto MC, Prigitano A, Lo Cascio G, et al. Yeast-like filamentous fungi: Molecular identification and in vitro susceptibility study. Med Mycol 2019;57:909-13. [Crossref] [PubMed]
- Pastor-Tudela AI, Pérez-González D, Jiménez-Montero B, et al. Catheter-related fungemia caused by Geotrichum capitatum in an immunocompetent pediatric patient. Enferm Infecc Microbiol Clin (Engl Ed) 2021;39:363-4. [Crossref] [PubMed]
- Miglietta F, Vella A, Faneschi ML, et al. Geotrichum capitatum septicaemia in a haematological patient after acute myeloid leukaemia relapse: identification using MALDI-TOF mass spectrometry and review of the literature. Infez Med 2015;23:161-7. [PubMed]
- Etienne A, Datry A, Gaspar N, et al. Successful treatment of disseminated Geotrichum capitatum infection with a combination of caspofungin and voriconazole in an immunocompromised patient. Mycoses 2008;51:270-2. [Crossref] [PubMed]



