
H1 and H2 antihistamines pretreatment for attenuation of protamine reactions after cardiopulmonary bypass: a randomized-controlled study
Highlight box
Key findings
• This study suggests that pretreatment with H1 and H2 antihistamines does not attenuate blood pressure responses to protamine administration in patients after CPB.
What is known and what is new?
• The mechanisms of protamine-induced cardiovascular reactions are not clearly understood, but are believed to be related to anaphylactic reactions, complement activation, and histamine release from mast cells.
• Our findings suggest that clinical doses of protamine administration may not induce mast cell degranulation in patients without risks for protamine allergy. Other mechanisms might be responsible for protamine-induced cardiovascular changes.
What is the implication, and what should change now?
• The clinical benefit of pretreatment with H1 and H2 antihistamines for protamine-related hemodynamic instability is uncertain, therefore, should not be recommended.
Introduction
Protamine is a basic protein extracted from fish sperm heads, which is the only standardized drug for heparin neutralization after cardiopulmonary bypass (CPB). Potential protamine reactions were first described in 1949 in dogs by Jaques et al. (1). Since then, there have been a number of reported cases of adverse hemodynamic effects of protamine in different clinical scenarios (2-9). The severity of hemodynamic reactions to protamine varies greatly, ranging from transient hypotension, pulmonary hypertension, impaired ventricular functions (5), and profound hypotension to fatal cardiac arrest (7,9,10). In a systematic review of serious protamine reactions by Nybo et al., which included a total of 27 different studies, they reported that the incidence of serious reactions ranged from 0.06% to 10.6% (11). Although severe adverse cardiovascular reactions are not common, protamine administration may induce systemic hypotension (drops in blood pressure >20%) in up to 52% of the patients (12).
Protamine-induced cardiovascular reactions can potentially lead to complications and greater risks of in-hospital mortality following cardiac surgery (12,13). Welsby et al. demonstrated that all degrees of systemic arterial hypotension and pulmonary artery hypertension during the first 30 minutes after protamine administration were independently associated with in-hospital mortality (12). Therefore, it is important to prevent hypotension after protamine administration. Although the mechanisms by which protamine produces adverse reactions are still not clearly understood, it is believed that intravascular administration of protamine exposes basophils and tissue mast cells to the compound resulting in mast cell degranulation and the release of histamines (14). Several studies have investigated the effects of pretreatment medications to minimize the cardiovascular effects of protamine (15,16). Antihistamines are one of the pretreatment drugs being examined (17-23). However, previous studies are outdated, published more than 20 years ago, and some are poorly designed. The results are heterogeneous and inconclusive because of the differences in the dosage of protamine administration, route of administration, and hemodynamic outcome measurements, which can be easily influenced by many factors.
Due to the inconsistency among the previously published studies (17-23), the primary aim of this study was to examine the effects of pretreatment H1 (chlorpheniramine) and H2 (ranitidine) antihistamines on changes in systemic arterial pressure following protamine administration in patients after CPB. We also evaluated systemic mast cell activity after protamine administration using serial serum tryptase levels. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-714/rc).
Methods
This study was a parallel-group, randomized, triple-blind, placebo-controlled study, conducted at Siriraj Hospital, a 2,200-bed quaternary-care medical center located in Bangkok, Thailand. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Siriraj Institutional Review Board (No. Si 518/2018) and was registered at clinicaltrail.gov (No. NCT03583567). Informed consent was obtained from all participants before recruitment. Patients aged ≥18 years old scheduled for elective coronary artery bypass grafting (CABG) or single valve surgery were included. Exclusion criteria were patients with a history of exposure to protamine-containing insulin, previous heart surgery, and history of allergy to either salmon or any of the study drugs.
Between October 2018 and March 2019, all patients scheduled for elective CABG or single valve surgery were screened for eligibility criteria and enrolled in the study (PW, CK). Blocks of 4 randomization was done using a computer-generated method. The randomization sequence was placed in opaque sealed envelopes separately with running numbers from 1 to 40. The patients were randomly allocated into two groups (1:1 ratio): group antihistamines (chlorpheniramine and ranitidine) and group NSS (normal saline). The participants, the attending anesthesiologists, and the outcome assessors were all blinded to group allocation.
Upon arrival in the operating room, standard noninvasive monitors were placed and midazolam 1 mg and dexmedetomidine 50 mcg were infused into all patients before arterial line insertion. Anesthesia was induced with propofol 1–2 mg/kg, fentanyl 1–2 mcg/kg, and cis-atracurium 0.02 mg/kg to facilitate intubation. Central venous catheter, urinary catheter, and nasopharyngeal temperature probe were placed after induction. Anesthesia was maintained with 1–2% sevoflurane in a mixture of air/O2, fentanyl 1 mcg/kg/hr, and cis-atracurium 0.03 mg/kg every 30 minutes throughout the operations. All medications that potentially trigger histamine release were avoided. Patients were ventilated to achieve normocarbia (PaCO2 of 35–45 mmHg). Systolic blood pressure (SBP) and mean arterial pressure (MAP) were maintained within 20% of the patient’s baseline before CPB was established. Heparin 3 mg/kg (300 U/kg) was given before aortic cannulation and activated clotting time (ACT) was maintained more than 480 seconds before CPB initiation and throughout CPB.
Management during CPB was based on our institutional standard. The bypass circuit was primed with acetate ringer’s solution 1,800 mL, heparin 5,000 U, sodium bicarbonate 44.6 mEq, 20% mannitol 0.5 g/kg, and 20% albumin 100 mL. CPB was performed with a non-occlusive roller pump and non-pulsatile flow was maintained between 2.0–2.4 L/min/m−2 with a target perfusion pressure of 50–80 mmHg during the CPB period. Intermittent bolus of norepinephrine was used to maintain perfusion pressure as needed if the target non-pulsatile flow was achieved. Mild hypothermia (32–34 °C) was instituted along with alpha-stat pH management. St. Thomas cardioplegia was used for myocardial protection in all patients. The blood cardioplegia was mixed at a 4:1 blood to crystalloid ratio and was delivered antegrade or retrograde until asystole was achieved.
During CPB, the enclosed envelopes with group allocation numbers were opened and the study drugs were prepared by one of the authors (PW, SS) who were not involved in patient care. Immediately after separation from CPB (the venous cannula was clamped), the study drugs were given according to group allocation. Each syringe was labeled as ‘study drug’. In group antihistamines, chlorpheniramine 10 mg and ranitidine 50 mg in two separate syringes were administered intravenously, while in group NSS, the patients received 2 mL of normal saline in a 3-mL syringe and 1 mL of normal saline in another 3-mL syringe identical to the study drugs. Five minutes after the study drugs were given, 3 mg/kg of protamine was given in 5 minutes infusion via peripheral line. Types and doses of inotropic and vasopressor drugs for hemodynamic support after separation from CPB were selected based on the attending anesthesiologist’s judgment to maintain MAP within 20% of baseline. Surgical manipulation of the heart was minimized after the starting of protamine infusion in order to avoid abrupt hemodynamic changes from surgical factors. Decannulation was performed once half of the dose of the protamine was given. After the procedures ended, all patients were transferred to the cardiac surgical intensive care unit intubated.
Outcomes
SBP, MAP, diastolic blood pressure (DBP), heart rate, and central venous pressure (CVP) were recorded every 1 minute for the first 20 minutes (T1, T2, T3, …, T20) and every 5 minutes until 35 minutes (T25, T30, T35) after protamine was initiated (T0). Baseline SBP, MAP, and DBP were recorded at the time of the beginning of protamine infusion. All changes in SBP and MAP after protamine administration were calculated as percent differences from baseline. The percent differences between blood pressures (SBP and MAP) before and after protamine administration = [blood pressure at x minute (Tx) – blood pressure at baseline (T0)/blood pressure at baseline (T0)] ×100%. If the patients developed hypotension (SBP <90 mmHg or MAP <65 mmHg), either norepinephrine 4 mcg or 10% calcium carbonate 1 g bolus would be given intravenously. The doses of inotropes, vasopressors, and calcium administered were documented. We also calculated vasoactive-inotropic score (VIS) (24) at each time point as vital signs were recorded until 35 minutes after protamine. VIS = dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100 × epinephrine dose (mcg/kg/min) + 50 × levosimendan dose (mcg/kg/min) + 10 × milrinone dose (mcg/kg/min) + 10,000 × vasopressin dose (units/kg/min) + 100 × norepinephrine dose (mcg/kg/min).
Serial serum tryptase levels were obtained at baseline (T0), 30 (T30), and 60 minutes (T60) after the beginning of protamine infusion. All blood samples were drawn via arterial line (after discarding 10 mL of blood) into the tube containing EDTA. The samples were centrifuged and then stored at −20 °C until assayed. Serum tryptase was measured by an enzyme-linked immunosorbent assay (ELISA) with Anti-Tryptase immunoCAP® (Unicap-Tryptase, Pharmacia-Sweden). Differences between serum tryptase levels at T30 and T60 from baseline were also calculated. Mast cell degranulation was considered if there was a significant elevation above the individual’s baseline tryptase level according to the formula: (baseline tryptase × 1.2) + 2 ng/mL (25).
Statistical analysis
From the previous study by Kambam et al. (18), the reported mean difference in MAP among the patients who received antihistamines and the control group was 15 mmHg. The primary outcome of this study was the differences in MAP between the two groups. Using a 2-sided type I error of 0.01 and 90% power, a sample size of 20 participants per group was estimated to test the difference in MAP of 15 and standard deviation (SD) of 12.
Study data were collected and managed using REDCap® electronic data capture tools hosted at Mahidol University. All data were processed using PASW Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp. Demographic and perioperative data were collected. Preoperative risk was accessed using the European System for Cardiac Operative Risk Evaluation (EuroSCORE II) (26) calculated using the online calculator (https://www.euroscore.org/index.php?id=17&lang=en). Continuous data such as age, weight, height, ejection fraction, EuroSCORE II, differences in blood pressure, and serum tryptase levels were reported as mean ± SD or median [interquartile range (IQR)]. Continuous data were compared using Student’s t-test or Mann-Whiney U test as appropriate. Categorical data such as gender, underlying disease, and types of surgery are presented as number (%) and were compared using chi-square test. Generalized estimating equations (GEE) with exchangeable correlation structure was used to assess the effect of protamine administration on changes in SBP and MAP during the first 35 minutes (20-time points) after protamine administration. Time was treated as a categorical variable. GEE model included protamine, time, and protamine-by-time interaction. For each outcome variable (i.e., SBP, MAP), two GEE models were fitted i.e., without and with adjustment for EuroSCORE II, CPB time, and VIS at each time point. Effects of protamine were reported as crude and adjusted mean difference, 95% CI, and P value at baseline, T10, and T30. A P value less than 0.05 was considered statistically significant.
Results
Between October 2018 and March 2019, 40 patients were enrolled (20 participants in each group) and completed the study (Figure 1). Demographic, clinical, and intraoperative characteristics are presented in Table 1. There were no significant differences in overall characteristics of the patients between the two groups, except for gender. More patients in group NSS were male (85%) compared to group antihistamines (50%) (P=0.02). There were also no differences in EuroSCORE II, types of surgery, duration of CPB and aortic cross-clamp time, and doses of heparin or protamine.
Table 1
| Variable | Group antihistamine (n=20) | Group NSS (n=20) | P value |
|---|---|---|---|
| Age (year) | 63.6±18.5 | 62.8±16.5 | 0.89 |
| Weight (kg) | 61.0±13.1 | 62.3±11.9 | 0.74 |
| Height (cm) | 159.1±7.4 | 163.1±7.6 | 0.10 |
| BMI (kg/m2) | 24.0±4.6 | 23.3±3.3 | 0.55 |
| Male | 10 (50%) | 17 (85%) | 0.02 |
| ASA-PS classification III:IV | 19:1 | 19:1 | 1.00 |
| EuroSCORE II* (%) | 2.25 (1.28, 3.26) | 1.63 (1.01, 2.54) | 0.11 |
| Underlying disease | |||
| Diabetes mellitus | 9 (45%) | 5 (25%) | 0.19 |
| Hypertension | 13 (65%) | 15 (75%) | 0.49 |
| Dyslipidemia | 14 (70%) | 12 (60%) | 0.51 |
| Serum creatinine (mg/dL) | 1.2±1.0 | 1.1±0.4 | 0.78 |
| Creatinine clearance† (mL/min) | 74.3±27.7 | 73.7±23.7 | 0.95 |
| Left ventricular ejection fraction (%) | 61.1±12.3 | 57.4±15.1 | 0.40 |
| Types of surgery | 0.31 | ||
| CABG | 15 (75%) | 12 (60%) | |
| Valve surgery | 5 (25%) | 8 (40%) | |
| Cardiopulmonary bypass time (min) | 122.0±42.7 | 123.1±37.5 | 0.93 |
| Aortic cross clamping time (min) | 81.3±21.2 | 86.7±31.7 | 0.57 |
| Protamine (mg) | 247.5±131.7 | 260.0±102.2 | 0.74 |
| Heparin (mg) | 186.8±44.6 | 188.0±39.4 | 0.93 |
| Calcium administration | 9 (45%) | 11 (55%) | 0.53 |
| Blood/blood products administered after cardiopulmonary bypass (mL) | 974±603 | 1081±434 | 0.26 |
| Baseline (T0) | |||
| Systolic blood pressure (mmHg) | 104.3±14.9 | 100.8±15.2 | 0.46 |
| Mean arterial pressure (mmHg) | 71.2±10.9 | 72.5±10.1 | 0.70 |
| VIS | 12.4±10.8 | 9.1±5.3 | 0.23 |
Data are presented as mean ± standard deviation, median (P25, P75) or number (%). *, the European System for Cardiac Operative Risk Evaluation II was calculated using online calculator provided at https://www.euroscore.org/index.php?id=17&lang=en; †, creatinine clearance was estimated using the Cockcroft-Gault equation. NSS, normal saline; BMI, body mass index; ASA-PS, American Society of Anesthesiologists Physical Status; CABG, coronary artery bypass grafting; VIS, vasopressor inotropic score.
Figures 2 and 3 demonstrate trajectory SBP and MAP from the beginning until 35 minutes after protamine infusion, respectively. Within 5 minutes after the initiation of protamine infusion (T1–T5), both mean SBP and MAP increased from baseline in both groups. Then, after 5 minutes (completion of protamine infusion), SBP and MAP decreased to within 10% of baseline values in both groups. The mean and 95% confidence intervals (CI) of unadjusted and adjusted (after adjustment for EuroSCORE II, CPB time, and VIS) trajectory SBP and MAP over the first 35 minutes after protamine administration are shown in Figure 2A,2B and Figure 3A,3B, respectively. There were no significant differences in both crude SBP (mean difference: 3.3 mmHg, 95% CI, −8.9 to 15.4 mmHg, P=0.60 and mean difference: −7.1 mmHg, 95% CI, −1.1 to 15.3 mmHg, P=0.09 at T10 and T30, respectively), and adjusted SBP (mean difference: 5.8 mmHg, 95% CI, −5.4 to 17.1 mmHg, P=0.31 and mean difference: −3.9 mmHg, 95% CI, −11.9 to 4.0 mmHg, P=0.33 at T10 and T30, respectively). There were also no significant differences in crude MAP (mean difference: 4.3 mmHg, 95% CI, −2.4 to 10.9 mmHg, P=0.21 and mean difference: −2.1 mmHg, 95% CI, −6.9 to 2.7 mmHg, P=0.39 at T10 and T30, respectively) and adjusted MAP (mean difference: 5.3 mmHg, −1.1 to 11.7 mmHg, P=0.11 and mean difference: −0.7 mmHg, 95% CI, −5.9 to 4.4 mmHg, P=0.78 at T10 and T30, respectively) between the two groups (Table 2).
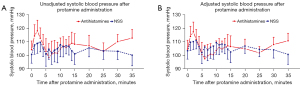
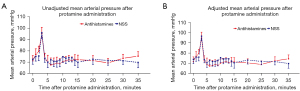
Table 2
| Variable | Crude | Adjusted† | |||
|---|---|---|---|---|---|
| Mean (95% CI) | P value | Mean (95% CI) | P value | ||
| Systolic blood pressure (mmHg) | |||||
| Baseline | −3.6 (−12.6, 5.6) | 0.44 | −1.2 (−10.4, 8.2) | 0.81 | |
| 10 minutes | 3.3 (−8.9, 15.4) | 0.60 | 5.8 (−5.4, 17.1) | 0.31 | |
| 30 minutes | −7.1 (−1.1, 15.3) | 0.09 | −3.9 (−11.9, 4.0) | 0.33 | |
| Mean arterial pressure (mmHg) | |||||
| Baseline | 1.3 (−5.0, 7.6) | 0.69 | 2.2 (−4.2, 8.6) | 0.50 | |
| 10 minutes | 4.3 (−2.4, 10.9) | 0.21 | 5.3 (−1.1, 11.7) | 0.11 | |
| 30 minutes | −2.1 (−6.9, 2.7) | 0.39 | −0.7 (−5.9, 4.4) | 0.78 | |
†, adjusted for EuroSCORE II, cardiopulmonary bypass time, and VIS. NSS, normal saline; EuroSCORE, European System for Cardiac Operative Risk Evaluation; VIS, vasopressor inotropic score; CI, confidence interval.
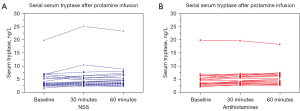
Figure 4 demonstrates individual’s serum tryptase levels at baseline, T30, and T60. None of the patients in both groups had a significant increase in serum tryptase levels from baseline (>20% +2 ng/mL). The median (IQR) serum tryptase at baseline, T30 and T60 were 4.1 (2.8) ng/mL, 4.3 (2.9) ng/mL, and 5.0 (3.2) ng/mL in group antihistamines, and 3.4 (3.2) ng/mL, 3.9 (3.1) ng/mL, and 4.5 (3.4) ng/mL in group NSS, respectively. There were no differences in serum tryptase levels at baseline, T30 and T60 between the two groups (P=0.49, P=0.49 and P=0.55 respectively). There was also no significant difference in changes in serum tryptase level from baseline between the two groups (P=0.62).
Discussion
The findings from this study indicate that pretreatment with intravenous H1 (chlorpheniramine) and H2 (ranitidine) antihistamines before protamine administration does not attenuate systemic blood pressure responses to protamine after CPB. There were no differences in trajectory blood pressures, vasopressor/inotropic uses, and serum tryptase levels in patients receiving pre-treatment antihistamines compared to the placebo. None of the patients in both groups had significant serum tryptase elevation from baseline suggesting that the clinical dose of protamine administration may not induce mast cell degranulation in patients without risks for protamine allergy. Hemodynamic changes following protamine administration might occur as a result of other mechanisms. Therefore, pretreatment with antihistamines did not modify the hemodynamic effects of protamine.
The mechanisms of protamine-induced hemodynamic reactions remain unclear. There are four types of adverse reactions being postulated (27,28). First, the classic anaphylactic reaction to the heparin-protamine complex via IgE-mediated, which rarely occurs but can cause severe abrupt onset cardiovascular collapse. Second, the anaphylactoid reaction involves activation of the complement system which results in systemic hypotension due to vasodilation. Third, complement-mediated C5a-induced thromboxane A2 generation is another mechanism that may lead to pulmonary vasoconstriction and systemic hypotension but is also uncommon. Finally, the most commonly believed mechanism is transient hypotension due to the histamine release from mast cells, which triggers nitric oxide (NO) production, a potent vasodilator, resulting in decreased systemic vascular resistance. Thus, antihistamines are one of the most common pretreatment drugs being investigated because of their safety profiles and availability worldwide. Antihistamine was also recommended by the Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA) guideline on patient blood management for adult cardiac surgery as a prophylactic drug to prevent hemodynamic perturbation following protamine administration (29). However, the evidence on the efficacy of pretreatment with antihistamines on cardiovascular reactions to protamine is not strong.
Table 3 presents a summary of the previous randomized clinical studies (RCT) investigating the effects of pretreatment antihistamines on cardiovascular and hemodynamic responses to protamine administration (17-23). All of the studies were old, published more than 20 years ago, and were small RCTs. Some of the studies divided the participants into several groups, hence, making it challenging to compare the changes in hemodynamics between groups (19,21). The studies did not mention placebo-controlled in the methods, hence, may be subject to bias. Furthermore, most of the studies only examined clinical hemodynamics, such as blood pressure, heart rate, and CVP (17-19,22,23), which might be influenced by several confounders, including, patient’s baseline conditions, volume status, surgical manipulation, and anesthetic agents. The study presented here was designed to examine the effects of combined H1 & H2 antihistamines pretreatment on systemic arterial pressure after protamine was administered. The authors also aimed to investigate whether the release of histamines from mast cells played a key role in hypotension after protamine. We chose serial serum tryptase levels as a surrogate for mast cell degranulation instead of serum histamine level because of the short half-life of histamine in the blood samples, which would make it difficult to properly handle the samples in the operating room before being transported to the laboratory.
Table 3
| Study | Population | Interventions | Protamine administration | Outcomes & conclusions | Remarks |
|---|---|---|---|---|---|
| Parsons 1989 (17) | 40 elective CABG, normotensive patients, good LVEF | 2 groups: no drug, H1 & H2 (chlorpheniramine & cimetidine) | Ratio 1:1, 10 minutes after CPB, via central line, rate 2.5 mg/sec (150 mg/min) | Lower BP and slower recovery in the control group; hemodynamic responses only be partially mediated by histamines | Rapid protamine administration via central venous line; follow parameters only the 5 minutes; 2 patients excluded due to hemodynamic instability |
| Kambam 1990 (18) | 30 elective CABG | 2 groups: no drug, H1 & H2 (diphenhydramine & cimetidine) | Ratio 1:2 (8 mg/kg of protamine), 5–8 minutes, via peripheral vein | 33% (control) vs. 0% (antihistamine) had decrease in BP >20% from baseline; prophylactic H1 and H2 antihistamines prevents hemodynamic side effects | High dose of protamine given; no placebo-control; compared maximum changes in hemodynamic parameters |
| Mayumi 1992 (19) | 126 [103] open heart surgery | 4 groups: no drug, H1 (diphenhydramine), H2 (famotidine), H1 & H2 (both) | N/A | Lower BP and slower recovery in the control group; only H2 antihistamine was effective | No placebo-control at first; further divided another 23 patients into 2 groups (famotidine and NSS) |
| Behne 1994 (20) | 20 elective CABG | 2 groups: no drug, H1 & H2 (dimetindene & ranitidine) | 350 u/kg protamine in 4 minutes, via peripheral vein | Comparable hemodynamics; no correlation between plasma histamine and hypotension; histamine may not play role in protamine reactions | Plasma histamine levels obtained until 10 minutes after protamine; small sample size; no placebo-control mentioned |
| Kanbak 1996 (21) | 40 cardiac surgery, normal LVEF | 4 groups: no drug H1 (terfenadine), H2 (ranitidine), H1 & H2 (both) | Based on ACT protamine dose assay worksheet, >3 minutes via peripheral vein | Plasma histamine-like activity (H-LA) increased after heparin not protamine; no difference in hemodynamic; histamine may not play role in protamine reactions | Small sample size; too many groups; excluded patients with sudden hemodynamic changes; follow parameters only 5 minutes |
| Lango 2000 (22) | 58 elective CABG, 5 (4 drugs, 1 control) patients excluded | 2 groups: no drug, H1 (clemestine) | Ratio 1:1.5, within 7 minutes via peripheral vein | Faster increase in BP to normal in clemestine group; clemestine pretreatment normalized BP after protamine | Per-protocol analysis; no placebo-control; compared parameters at the same interval, not trajectory |
| Sikander 2008 (23) | 60 open heart surgery (unspecified types) | 2 groups: no drug, H1 (chlorpheniramine) | Ratio 1:1.3 protamine >10 minutes i.v. (site not mentioned) | Higher BP and reduced inotropic requirement in the antihistamine group; antihistamine pretreatment helps maintain hemodynamic after protamine | Demographic data of the patients are not presented; no placebo-control; no statistical analysis explained |
CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; BP, blood pressure; NSS, normal saline; ACT, activated clotting time.
In this study, we could not demonstrate the differences in trajectory SBP and MAP during the first 30 minutes after protamine infusion between pretreatment with antihistamines and the control groups. After adjusted for EuroSCORE II, CPB time, and vasopressors and inotropic uses, the results remained the same. The findings are consistent with that reported by some of the previous studies (20,21). Kanbak et al. (21) did an RCT in 40 cardiac patients and compared between patients receiving prophylactic H1 (oral terfenadine), H2 (oral ranitidine), and both H1 & H2 antihistamines before operation compared to the control group. They reported no significant differences in hemodynamic variables within the 5 minutes after protamine infusion between any of the groups and the control group. Another study by Behne et al. also reported no difference in hemodynamic profiles between the group with pretreatment H1 & H2 antihistamines and the control in 20 patients who underwent elective CABG (20). In their study, besides hemodynamic parameters, serial serum histamine levels until 10 minutes after protamine were also examined. A slight increase in serum histamine levels was observed in both groups, however, there was no association between the slightly elevated histamine levels and clinical hypotension. The authors concluded that histamine release may not be the underlying mechanism of protamine-related hypotension.
In contrast to our findings, some of the previous studies had demonstrated the benefit of pretreatment with antihistamines in attenuating hemodynamic changes following protamine administration (17-19,22,23). Lango et al. reported a more rapid recovery in systemic blood pressure after drops following protamine in patients pretreated with H1 antihistamine (clemastine) compared to the control group in 53 patients who underwent CABG (22). However, four patients in the antihistamine group and one patient in the control group were excluded from the analysis after randomization due to severe hemodynamic instability. Moreover, the differences in SBP, MAP, and DBP reported in this study were only about 5–10 mmHg, which may be considered insignificant clinically. Another study by Parsons et al. in 38 patients with good left ventricular ejection fraction who underwent elective CABG, demonstrated a more subtle decline in blood pressures from baseline in the antihistamine group (−25%) compared to the control group (−32%) (17). In their study, dramatic falls in blood pressure were observed in both groups following protamine administration since protamine was rapidly injected (2.5 mg/sec) via central venous catheter, while in our study, we slowly administered protamine in 5 minutes via peripheral line. A faster rate of administration of protamine resulted in a more severe hemodynamic perturbation than slow administration (6,30). Furthermore, the patients did not receive any vasopressor and inotropic support after separation from CPB, therefore, the magnitude of hemodynamic effects of protamine was more prominent than in our study which hypotension was partially treated with vasopressor and inotropic medications. In this study, an increase in blood pressure from baseline (T0) was observed during the first few minutes after separation from CPB, which might be due to the restoration of preload and effects from inotropic/vasopressor titration, followed by a reduction of blood pressure to baseline level after the completion of protamine infusion in both groups. During the first few minutes of protamine infusion, the serum concentration of protamine had not reached its peak, hence, prominent hemodynamic effects from protamine were not observed. We used a fixed 1:1 ratio (1 mg protamine to every 100 IU of heparin) because this ratio was still commonly used in our institute when we started the recruitment and was a common recommendation (31). Despite the dose used in our study may have caused excess free drug compared to the lower dose (ratios of 0.6 or 0.8) as recommended by current evidence, we could not demonstrate any difference in systemic blood pressure after protamine was given. This suggests that pretreatment with antihistamines should not be useful in lower dose ranges of protamine as well. Unlike our study, the statistical differences in blood pressures between groups being reported in most of the positive studies were only minor (<10 mmHg), which might not be clinically meaningful and should be manageable with a slow rate of protamine administration and low-dose vasopressors. Hence, the clinical benefit of pretreatment with antihistamines for attenuating protamine-related hemodynamic instability is uncertain.
Regarding serial serum tryptase levels, none of the patients in both groups had significant elevation in serial serum tryptase within 60 minutes after protamine exposure, suggesting that protamine reactions might not be mediated through mast cell degranulation, in patients without anaphylaxis. An anaphylactic reaction is rare and we also excluded patients with potential risks for protamine allergy, such as a history of exposure to protamine-containing insulin, previous heart surgery, and history of allergy to salmon. An in vitro study demonstrated that protamine caused dose-related histamine release from human skin mast cells but only when much higher concentrations than that in clinical practice were used (32). A previous study by Kambam et al. demonstrated a significant decline in blood pressures (MAP –15 mmHg) after protamine in the control group compared to stable blood pressures in the group with antihistamines pretreatment (18). In their study, 8 mg/kg of protamine was used, which was much higher than the dose currently used nowadays. While in our study, only 3 mg/kg of protamine was used and slowly administered, therefore, the level of protamine might not reach intravascular concentrations which trigger the histamine release.
Mechanisms other than histamine release from mast cells have also been elucidated. First is the inflammatory response induced by C-reactive protein resulting in complement activation through the classical C4a pathway (33). In a prospective observational study examining the incidence of protamine reactions among 243 patients who underwent surgery using CPB, they reported an increase in C4a levels in all groups of patients after protamine administration and significantly higher levels in the group with reactions (34). Another possible mechanism for hypotension following protamine is vasoplegia, which has been demonstrated in vitro (14). Protamine activates endothelial NO synthase pathway resulting in vasorelaxation demonstrated in the isolated internal thoracic artery (35). NO is a potent vasodilator and also inhibits platelet aggregation. Moreover, a study reported that protamine augmented intracellular endothelial Ca2+ release in response to mechanical stretch, causing vasodilatation in the isolated human umbilical vein cells (36). In a small study among 22 patients after CPB, protamine neutralization of heparin resulted in gradual drops in systemic vascular resistance and blood pressure eventually decreased once cardiac output was over-compensated (2). Lastly, a recent in vitro study using isolated mitochondria from rat hearts demonstrated that protamine caused an excessive increase in mitochondrial reactive oxygen species (ROS), resulting in mitochondrial dysfunction (37). The authors proposed that excessive formation of ROS might be the pathogenesis of the cardiovascular (both systemic hypotension and pulmonary hypertension) side effects of protamine. All in all, the findings from our study supported that mast cell degranulation might not play a major role in the unwanted cardiovascular side effects of protamine and other mechanisms should rather be taken into account and further investigated.
There are some limitations to this study. First, the small sample size may increase the likelihood of type II error. Secondly, the primary outcome of this study was the differences in MAP between the two groups. Although we tried to analyze the differences in blood pressures after adjusted for EuroSCORE II, CPB time, and VIS score at each time point, however, blood pressures can also be influenced by many factors, such as volume status, ventricular functions, vasoplegic state, surgical bleeding, and surgical manipulation. It can be challenging to control and compare these factors, hence, may be a confounder to our study. Although we tried to include only the patients who underwent similar procedures (elective CABGs and single valve surgery), it was not possible to control the differences in the complexity of the procedures and CPB time of each individual, which may influence hemodynamics during post-CPB. Lastly, we excluded patients with risks for protamine allergy, such as a history of exposure to protamine-containing insulin, previous heart surgery, and a history of allergy to salmon, therefore, the negative results of pretreatment antihistamines from our study might not be applicable and antihistamines may still be beneficial for these patients.
Conclusions
In conclusion, pretreatment with H1 and H2 antihistamines does not attenuate blood pressure responses to protamine administration in patients after CPB. Moreover, none of the patients in our study had significant elevation in serial serum tryptase levels, thus suggesting that it is not common for protamine administration to cause systemic mast cell degranulation and histamine release in patients without allergy. Other mechanisms might be responsible for protamine-induced cardiovascular changes after protamine administration.
Acknowledgments
The authors would like to express their gratitude to Miss Chulaluk Komoltri for statistical analysis, and Miss Nichapat Sooksri for her help in organizing this study.
Funding: This research project was supported by Siriraj Foundation Funding, Faculty of Medicine Siriraj Hospital, Mahidol University (No. D003903).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-714/rc
Trial Protocol: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-714/tp
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-714/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-714/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Siriraj Institutional Review Board (No. Si 518/2018) and was registered at clinicaltrail.gov (No. NCT03583567). Informed consent was obtained from all participants before recruitment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jaques LB. A study of the toxicity of the protamine, salmine. Br J Pharmacol Chemother 1949;4:135-44. [Crossref] [PubMed]
- Shapira N, Schaff HV, Piehler JM, et al. Cardiovascular effects of protamine sulfate in man. J Thorac Cardiovasc Surg 1982;84:505-14. [Crossref] [PubMed]
- Lowenstein E, Zapol WM. Protamine reactions, explosive mediator release, and pulmonary vasoconstriction. Anesthesiology 1990;73:373-5. [Crossref] [PubMed]
- Gourin A, Streisand RL, Greineder JK, et al. Protamine sulfate administration and the cardiovascular system. J Thorac Cardiovasc Surg 1971;62:193-204. [Crossref] [PubMed]
- Del Re MR, Ayd JD, Schultheis LW, et al. Protamine and left ventricular function: a transesophageal echocardiography study. Anesth Analg 1993;77:1098-103. [Crossref] [PubMed]
- Michaels IA, Barash PG. Hemodynamic changes during protamine administration. Anesth Analg 1983;62:831-5. [Crossref] [PubMed]
- Leung LWM, Gallagher MM, Evranos B, et al. Cardiac arrest following protamine administration: a case series. Europace 2019;21:886-92. [Crossref] [PubMed]
- Nielsen VG, Kazui T, Horn EA, et al. Thrombocytosis and neutrophilia associated with oxygenator failure and protamine reaction after cardiopulmonary bypass: a case report and literature review. J Thromb Thrombolysis 2021;52:1220-6. [Crossref] [PubMed]
- Peeters M, Yilmaz A, Vandekerkhof J, et al. Protamine Induced Anaphylactic Shock after Peripheral Vascular Surgery. Ann Vasc Surg 2020;69:450.e13-5. [Crossref] [PubMed]
- Sokolowska E, Kalaska B, Miklosz J, et al. The toxicology of heparin reversal with protamine: past, present and future. Expert Opin Drug Metab Toxicol 2016;12:897-909. [Crossref] [PubMed]
- Nybo M, Madsen JS. Serious anaphylactic reactions due to protamine sulfate: a systematic literature review. Basic Clin Pharmacol Toxicol 2008;103:192-6. [Crossref] [PubMed]
- Welsby IJ, Newman MF, Phillips-Bute B, et al. Hemodynamic changes after protamine administration: association with mortality after coronary artery bypass surgery. Anesthesiology 2005;102:308-14. [Crossref] [PubMed]
- Kimmel SE, Sekeres M, Berlin JA, et al. Mortality and adverse events after protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg 2002;94:1402-8. table of contents. [Crossref] [PubMed]
- Boer C, Meesters MI, Veerhoek D, et al. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth 2018;120:914-27. [Crossref] [PubMed]
- Alqassieh R, Odeh M, Al-Sabbagh MQ, et al. The role of hydrocortisone pre-treatment in decreasing side effects of protamine sulfate administration during cardiac surgery: a randomised controlled trial. Perioperative Care and Operating Room Management 2021;23:100161. [Crossref]
- Baraka A, Choueiry P, Taha S, et al. Hydrocortisone pretreatment for attenuation of protamine-induced adverse hemodynamic reactions. J Cardiothorac Vasc Anesth 1995;9:481-2. [Crossref] [PubMed]
- Parsons RS, Mohandas K. The effect of histamine-receptor blockade on the hemodynamic responses to protamine. J Cardiothorac Anesth 1989;3:37-43. [Crossref] [PubMed]
- Kambam J, Meszaros R, Merrill W, et al. Prophylactic administration of histamine1 and histamine2 receptor blockers in the prevention of protamine-related haemodynamic effects. Can J Anaesth 1990;37:420-2. [Crossref] [PubMed]
- Mayumi H, Toshima Y, Tokunaga K. Pretreatment with H2 blocker famotidine to ameliorate protamine-induced hypotension in open-heart surgery. J Cardiovasc Surg (Torino) 1992;33:738-45. [PubMed]
- Behne M, Bremerich D, Schiesser S, et al. Effectiveness of preventing hypotension with H1/H2 antagonists before protamine administration. Infusionsther Transfusionsmed 1994;21:81-5. [PubMed]
- Kanbak M, Kahraman S, Celebioglu B, et al. Prophylactic administration of histamine 1 and/or histamine 2 receptor blockers in the prevention of heparin- and protamine-related haemodynamic effects. Anaesth Intensive Care 1996;24:559-63. [Crossref] [PubMed]
- Lango R, Mroziński P, Wujtewicz M, et al. Administration of clemastine--H1 histamine receptor blocker in the prevention of haemodynamic disorders after protamine sulfate administration in patients subjected to coronary artery bypass grafting in extracorporeal circulation. Med Sci Monit 2000;6:769-75. [PubMed]
- Sikander RI. Role of Antihistamines in Preventing Haemodynamic Deterioration Caused by Protamine Sulphate in Cardiac Surgery: A Randomized Control Study. Ann Pak Inst Med Sci 2008;4:232-6.
- Koponen T, Karttunen J, Musialowicz T, et al. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth 2019;122:428-36. [Crossref] [PubMed]
- Baretto RL, Beck S, Heslegrave J, et al. Validation of international consensus equation for acute serum total tryptase in mast cell activation: A perioperative perspective. Allergy 2017;72:2031-4. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Brück S, Skrabal C, Träger K, et al. Risk factors for adverse reactions after protamine administration in adult patients undergoing cardiac surgery- a case report and literature review. Anasthesiol Intensivmed Notfallmed Schmerzther 2014;49:360-6. [PubMed]
- Chaney MA, Devin Roberts J, Wroblewski K, et al. Protamine Administration Via the Ascending Aorta May Prevent Cardiopulmonary Instability. J Cardiothorac Vasc Anesth 2016;30:647-55. [Crossref] [PubMed]
- Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:79-111. [Crossref] [PubMed]
- Wakefield TW, Hantler CB, Wrobleski SK, et al. Effects of differing rates of protamine reversal of heparin anticoagulation. Surgery 1996;119:123-8. [Crossref] [PubMed]
- Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg 2020;57:210-51. [PubMed]
- Sauder RA, Hirshman CA. Protamine-induced histamine release in human skin mast cells. Anesthesiology 1990;73:165-7. [Crossref] [PubMed]
- Bruins P, te Velthuis H, Eerenberg-Belmer AJ, et al. Heparin-protamine complexes and C-reactive protein induce activation of the classical complement pathway: studies in patients undergoing cardiac surgery and in vitro. Thromb Haemost 2000;84:237-43. [Crossref] [PubMed]
- Weiler JM, Gellhaus MA, Carter JG, et al. A prospective study of the risk of an immediate adverse reaction to protamine sulfate during cardiopulmonary bypass surgery. J Allergy Clin Immunol 1990;85:713-9. [Crossref] [PubMed]
- Pevni D, Gurevich J, Frolkis I, et al. Protamine induces vasorelaxation of human internal thoracic artery by endothelial NO-synthase pathway. Ann Thorac Surg 2000;70:2050-3. [Crossref] [PubMed]
- Murase K, Naruse K, Kimura A, et al. Protamine augments stretch induced calcium increase in vascular endothelium. Br J Pharmacol 2001;134:1403-10. [Crossref] [PubMed]
- Ramzan R, Michels S, Weber P, et al. Protamine Sulfate Induces Mitochondrial Hyperpolarization and a Subsequent Increase in Reactive Oxygen Species Production. J Pharmacol Exp Ther 2019;370:308-17. [Crossref] [PubMed]



