
Nomogram for prediction of portal vein system thrombosis after splenectomy for hypersplenism in patients with Wilson disease
Introduction
Wilson disease (WD) is a recessive hereditary disease that is a disorder of copper metabolism caused by an ATP7B gene mutation (1). Excess copper is pathologically deposited in the liver, brain, kidney, and other tissues. Epidemiological studies have shown that the global prevalence of WD is about 0.25 to 4 per 10,000 people (2), and the main clinical manifestations are liver disease and neurological symptoms. Fibrosis is the main pathological change in the liver of patients with WD, which can progress to cirrhosis. Advanced liver cirrhosis can lead to symptoms such as portal hypertension, splenomegaly, and hypersplenism. Splenectomy is an effective treatment for cirrhotic hypersplenism. However, after splenectomy, the risk of portal vein system thrombosis (PVST) increases significantly (3). PVST can lead to a variety of complications, such as gastrointestinal bleeding, hepatic encephalopathy, and intestinal obstruction, seriously affecting the prognosis of patients and even threatening their lives (4). Most patients with PVST lack typical clinical symptoms and signs in the early stages and are easily overlooked (5). Therefore, early detection of patients with high-risk WD after splenectomy and timely intervention are particularly important.
Presently, relevant studies are primarily concerned with discussing the risk factors of PVST after splenectomy for hypersplenism in patients with WD (6,7), and there are few studies investigating a prediction model or nomogram of PVST after splenectomy in these patients. Therefore, the main purpose of this study was to establish and validate a nomogram for the risk of PVST after splenectomy in patients with WD that would enable clinicians to identify high-risk patients as early as possible. We present the following article in accordance with the TRIPOD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-826/rc).
Methods
Patients
The clinical data of patients with WD and liver cirrhosis who underwent splenectomy from January 2019 to March 2022 at the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine were retrospectively included in this study.
The inclusion criteria for patients were defined as follows: (I) diagnosis of Wilson disease (WD) and liver cirrhosis with secondary hypersplenism; (II) moderate to high degree of spleen enlargement; (III) no ascites or a small amount of ascites; (IV) normal or almost normal liver function with corrected hypoalbuminemia; (V) pancytopenia of 1 or more of the whole blood cells, especially white blood cell count (WBC) ≤30×109/L or (and) platelet count (PLT) <60×109/L; (VI) proliferative bone marrow on bone marrow examination, (VII) Child-Pugh liver function classification of A or B; and (VIII) abdominal color Doppler ultrasound examination before and after splenectomy. The exclusion criteria for patients included the following: (I) chronic liver disease of other causes (alcohol, autoimmunity, drugs, etc.), (II) combination of malignant tumors and severe systemic diseases, (III) long-term use of antiplatelet and anticoagulant drugs, and (IV) preoperative presence of PVST. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (No. 2022MCZQ18) and individual consent for this retrospective analysis was waived.
Perioperative examination and splenectomy
The routine perioperative examination included routine blood tests, liver function tests, coagulation function tests, and abdominal color Doppler ultrasound. Splenectomy can effectively improve the symptoms of portal hypertension and hypersplenism in patients with WD. The preoperative diagnosis was based on Guidelines for the Diagnosis and Treatment of Hepatolenticular Degeneration (2022) (8) formulated by the Genetic and Metabolic Liver Diseases Collaborative Group of the Chinese Medical Association’s Hepatology Branch. The precision splenectomy began first with ligation of the splenic artery so that the splenic blood was fully transfused. The spleen was then brought close to a bloodless state, and one-by-one, the grade 2 to 3 splenic blood vessels were ligated. Finally, wound hemostasis was achieved and suturing completed. The PLT and color Doppler ultrasound of the portal venous system were monitored dynamically after the operation. The patients with splenectomy were all treated with prophylactic anticoagulation, and there was no obvious bleeding tendency within 3 days after surgery. When platelets were significantly elevated (>500×109/L), anticoagulation therapy consisting of a subcutaneous injection of low–molecular–weight heparin calcium injection (4,000 IU q12h) was administered for 7 to 10 days, and 200,000 units of urokinase were added to the intravenous infusion when PVST appeared. After reexamination of the color ultrasound, it was found that the PVST had disappeared, and the PLT had returned to normal. The low–molecular–weight heparin calcium injection and urokinase were discontinued, and oral aspirin anticoagulation was administered for 3–6 months. The results of routine blood tests, liver and kidney function tests, and coagulation tests were closely monitored during treatment to assess the bleeding risk.
Clinical variables
The clinical variables in this study are reported in Table 1. According to relevant the literature (4,9), the clinical data of the patients were collected. The preoperative data included gender, age, Child-Pugh score, WBC, red blood cell count (RBC), PLT, bilirubin level, and presence of ascites within 1 week before splenectomy. The postoperative data included WBC, RBC, PLT, prothrombin time (PT), activated partial thromboplastin time (APTT), international standard ratio (INR), D-dimer, mean platelet volume (MPV), mean platelet distribution width (PDW) within 1 day after splenectomy, PLT increase (the difference between the mean PLT within 1 week after the operation and the preoperative PLT), and operation time.
Table 1
| Variables | PVST (n=50) | Non-PVST (n=31) | t/Z/χ2 | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 31.46±14.10 | 30.77±11.60 | −0.019 | 0.818 |
| Gender [n (%)] | 0.044 | 0.834 | ||
| Male | 27 (54.0) | 16 (51.6) | ||
| Female | 23 (46.0) | 15 (48.4) | ||
| Child-Pugh [n (%)] | 0.209 | 0.648 | ||
| A | 30 (60.0) | 17 (54.8) | ||
| B | 20 (40.0) | 14 (45.2) | ||
| Ascites [n (%)] | 0.046 | 0.831 | ||
| Yes | 15 (30.0) | 10 (32.3) | ||
| No | 35 (70.0) | 21 (67.7) | ||
| Portosystemic devascularization [n (%)] | <0.001 | 0.988 | ||
| Yes | 8 (16.0) | 5 (16.1) | ||
| No | 42 (84.0) | 26 (83.9) | ||
| Operation time (min) | 136 [119, 170] | 142 [120, 171] | −0.506 | 0.613 |
| Preoperative | ||||
| Diameter of portal vein (mm) | 13 [12, 15] | 12 [11, 13] | 2.866 | 0.004 |
| Portal blood flow velocity (cm/s) | 15.85 (13.80, 18.13) | 19.90 (15.80, 22.70) | −3.523 | <0.001 |
| Total bilirubin (μmol/L) | 18.53 (13.37, 25.08) | 20.00 (14.32, 25.73) | −0.437 | 0.662 |
| WBC (×109/L) | 2.28 (1.73, 2.97) | 2.57 (1.93, 3.66) | −1.545 | 0.122 |
| RBC (×1012/L) | 4.06±0.58 | 3.84±0.58 | 1.647 | 0.104 |
| PLT (×109/L) | 48.50 (34.75, 59.25) | 41.00 (35.00, 55.00) | 0.578 | 0.563 |
| Postoperative | ||||
| WBC (×109/L) | 15.94 (11.45, 19.18) | 16.02 (12.92, 21.59) | −0.670 | 0.503 |
| RBC (×1012/L) | 4.45±0.70 | 4.14±0.70 | −1.857 | 0.067 |
| PLT (×109/L) | 117.00 (92.75, 141.25) | 100.00 (88.00, 124.00) | −1.317 | 0.188 |
| MPV (fL) | 11.78±0.92 | 12.22±0.85 | 2.181 | 0.032 |
| PDW (fL) | 15.00 (12.98, 17.55) | 15.80 (13.90, 19.00) | −1.992 | 0.046 |
| D-dimer (mg/L) | 12.76 (9.72, 14.95) | 8.22 (4.57, 10.52) | −3.703 | <0.001 |
| PT (sec) | 13.72±2.12 | 12.85±1.51 | −1.996 | 0.049 |
| APTT (sec) | 33.79±5.78 | 34.81±8.62 | 0.635 | 0.527 |
| INR | 1.17 (1.07, 1.29) | 1.21 (1.14, 1.30) | −1.293 | 0.196 |
| PLT increase (×109/L) | 131.00 (95.88, 171.00) | 95.00 (73.00, 120.00) | 3.148 | 0.002 |
PVST, portal vein system thrombosis; WBC, white blood cell count; RBC, red blood cell count; PLT, platelet count; MPV, mean platelet volume; PDW, mean platelet distribution width; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international standard ratio; PLT increase, the difference between the mean PLT within 1 week after the operation and the preoperative PLT.
Statistical methods
Statistical analysis was performed using SPSS v. 26.0 (IBM Corp., Armonk, NY, USA) and R v. 4.1.3 software (http://www.R-project.org). We used univariate analysis to evaluate the correlation between each index and PVST. Continuous numerical variables that followed a normal distribution are expressed as mean ± standard deviation () and were compared using a t-test. The Mann-Whitney test was used to compare nonnormal distributions, which are expressed as median (interquartile range). Categorical variables were compared using the chi-square (χ2) test and are expressed as proportions, numbers, and percentages The least absolute shrinkage and selection operator (LASSO) regression analysis was used to reduce high-dimensional data and to screen independent risk factors for PVST from clinical indicators. Multivariate logistic regression analysis was used to establish a nomogram. A value of P<0.05 was considered statistically significant. Internal validation was undertaken using the bootstrap method (1,000 bootstraps resamples). R software was used to draw the nomogram, calculate the concordance (C)-index, and draw the model calibration curve, receiver operating characteristic (ROC) curve, and clinical decision analysis (DCA) curve.
Results
Characteristics
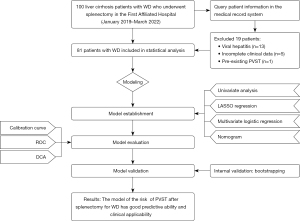
A total of 100 patients with WD and hypersplenism were retrospectively included in the study. After exclusion of those with viral hepatitis (n=13), incomplete clinical data (n=5), or pre-existing PVST (n=1), 81 eligible patients were finally included (Figure 1). The incidence of PVST was 61.7%. There were 31 patients without PVST (16 males, 15 females; mean age 30.77 years) and 50 patients with PVST (27 males, 23 females; mean age 31.46 years), including 21 patients with portal vein thrombosis, 12 patients with splenic vein thrombosis, and 16 patients with both portal vein and splenic vein thrombosis. Of these, 36 patients were diagnosed with PVST on day 7, 12 patients were diagnosed with PVST in week 2, and only 1 patient was diagnosed with PVST after 2 weeks. No statistical difference was found between the 2 groups with respect to age or gender (P>0.05; Table 1).
Univariate analysis
Univariate analysis was applied to the clinical data of patients in the PVST group and the non-PVST group. The results showed that widening of the preoperative portal vein diameter and a decrease in the velocity of portal blood flow were risk factors for PVST formation after splenectomy. Differences in the postoperative indicators, MVP, PDW, D-dimer, PT, and PLT increase were also statistically significant (P<0.05; Table 1). Univariate logistic analysis was used for comparing the velocity of postoperative portal blood flow in patients who developed PVST after 1 week with patients in the non-PVST group. The results showed that a decrease in the velocity of postoperative portal blood flow was one of the risk factors for the occurrence of PVST (P<0.05; Table 2).
Table 2
| Variables | β | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Portal blood flow velocity (cm/s) | −0.222 | 0.107 | 4.306 | 0.801 | 0.649–0.988 | 0.038 |
| Constant | 2.593 | 1.633 | 2.522 | 13.365 | – | 0.112 |
β, regression coefficients; SE, standard error; OR, odds ratio; CI, confidence interval.
Features selection
The features with statistically significant differences (P<0.05) in the univariate analysis were included in the LASSO regression analysis to screen out features of the nonzero coefficient, and cross-validation was used to determine suitable optimal parameters (lambda [λ]; Figure 2). The results yielded a λ value of 7, indicating that all 7 features included in LASSO were important.

Multivariate logistic regression analysis
The results of multivariate logistic regression analysis showed that preoperative portal vein diameter, velocity of preoperative portal blood flow, postoperative D-dimer, and PLT increase were independent risk factors for PVST after splenectomy in patients with WD (Table 3).
Table 3
| Variables | β | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Diameter of portal vein (mm) | 0.293 | 0.146 | 4.046 | 1.341 | 1.008–1.784 | 0.044 |
| Portal blood flow velocity (cm/s) | −0.144 | 0.073 | 3.907 | 0.866 | 0.759–0.999 | 0.048 |
| PLT increase (×109/L) | 0.015 | 0.006 | 6.173 | 1.016 | 1.003–1.028 | 0.013 |
| D-dimer (mg/L) | 0.137 | 0.066 | 4.347 | 1.147 | 1.008–1.304 | 0.037 |
| Constant | −4.042 | 2.602 | 2.414 | 0.018 | – | 0.120 |
PVST, portal vein system thrombosis; WD, Wilson disease; β, regression coefficients; SE, standard error; OR, odds ratio; CI, confidence interval; PLT, platelet count.
Establishment of the personalized nomogram
Using multivariate logistic regression analysis, we identified 4 independent risk factors for predicting PVST after splenectomy in patients with WD, and incorporated these into the nomogram (Figure 3).

Evaluation of the accuracy and predictive power of the nomogram model
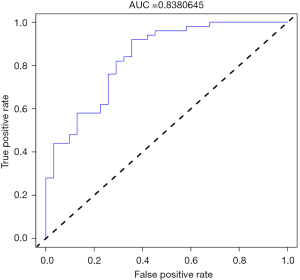
By drawing the ROC curve of the population, the area under the ROC (AUC) of the model was calculated as 0.838 (95% CI: 0.750–0.926) (Figure 4) and the C-index was calculated as 0.838. Internal validation by the bootstrap method also showed a moderate C-index of 0.805. Thus, the PVST risk nomogram demonstrated good discrimination and good predictive ability. The calibration curve showed that the line of the nomogram was close to the ideal model, indicating that the model was appropriate (Figure 5).

Evaluation of clinical applicability
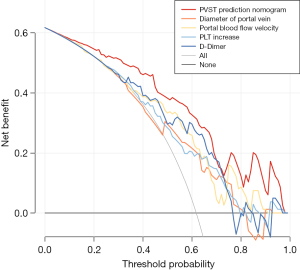
The DCA curve showed that when the nomogram predicts the probability of PVST in patients with WD after splenectomy to be 2–100%, using this model to predict and identify the occurrence of PVST and taking corresponding treatment measures can make patients benefit clinically. The net benefit rate was higher than the prediction of PVST using a single risk factor (Figure 6), indicating that the model had good clinical applicability.
Discussion
WD is a recessive hereditary disease caused by an ATP7B gene mutation (1). When the ATP7B gene mutation leads to dysfunction of the protein that transports copper, a large amount of copper accumulates in the liver to cause abnormal liver structure with potential progression to cirrhosis (10), leading to portal hypertension and secondary hypersplenism. PVST refers to the occurrence of thrombus in the portal vein, splenic vein, superior mesenteric vein, or intrahepatic portal vein (11). It is one of the complications in patients with hypersplenism who undergo splenectomy.
Research has shown that splenectomy for cirrhosis is associated with a higher prevalence of PVST than that found in nonsurgical patients (3,5), and the incidence of PVST after splenectomy in patients with WD in our study was about 62%. PVST is often overlooked in the early stage of its formation due to the absence of specific symptoms and signs (5). Failure to intervene in time after thrombosis can lead to gastrointestinal congestion and edema. In severe cases, refractory ascites may occur, and the reduction of hepatic blood flow can aggravate liver damage, leading to liver failure and even death (4). Therefore, it is particularly important to predict the risk of PVST after splenectomy for patients with WD and hypersplenism in a timely manner and carry out effective treatment.
At present, the risk factors for PVST formation after splenectomy in patients with liver cirrhosis include increased portal vein diameter, splenic vein diameter, PLT, bilirubin level and postoperative D-dimer, and decreased postoperative MPV (12,13). In all, 81 patients with WD and hypersplenism were retrospectively included in our study. Through univariate analysis, LASSO analysis, and multivariate logistic regression analysis, we found that preoperative portal vein diameter increase, preoperative portal blood flow velocity reduction, postoperative D-dimer elevation, and PLT increase are independent risk factors for PVST after splenectomy in patients with WD and cirrhosis complicated by hypersplenism. In addition, we found that all patients with preoperative collateral circulation developed PVST after splenectomy. Since only 10 patients in this study had preoperative collateral circulation, including the esophagogastric fundus, hepatic porta, splenic porta, and umbilical vein, we did not include this index in the prediction model, but we intend to undertake further study of it in the future. In contrast, gender, age, and Child-Pugh score were not found to be independent risk factors for PVST after splenectomy in patients with WD and cirrhosis complicated by hypersplenism.
The “three elements” of PVST formation in patients with liver cirrhosis after splenectomy include hemodynamic changes in the portal venous system, blood hypercoagulability, and vascular endothelial damage (9,14-16). Portal vein diameter and velocity of portal blood flow can reflect the hemorheological alterations. This study found that increased portal vein diameter was positively correlated with the formation of PVST, while the decreased of portal blood flow velocity was negatively correlated with the formation of PVST. The odds ratio (OR) values were 1.341 and 0.866, respectively. These findings suggest that the larger the diameter of the portal vein is and the slower the velocity of portal blood flow, the greater is the risk of PVST after splenectomy. One study has shown that when the diameter of the portal vein widens and the velocity of blood flow slows down, eddy currents are easily formed in the blood. This leads to an increase in the contact time between platelets and fibrinogen in the blood and the inner wall of the blood vessel and increases the concentration of coagulation factors, thereby increasing the risk of thrombosis (17). Persistent portal hypertension acting on the vascular wall and surgery can cause vascular endothelial damage and exposure to collagen fibers, leading to platelet aggregation and thrombosis (18). Postoperative local inflammation of blood vessels stimulates damaged blood vessels and also promotes platelets to aggregate and, thus, cause thrombosis (19). After splenectomy, the numbers of red blood cells, white blood cells, and platelets increase significantly in a short period, making the blood turn into a hypercoagulable state. In particular, the significant increase in PLT increases blood viscosity. This study found that the OR of increased PLT and postoperative D-dimer were 1.016 and 1.147, respectively, indicating that they were positively correlated with the risk of PVST formation. This suggests that the higher the increase in PLT and postoperative D-dimer is, the higher is the risk of PVST.
In this study, a predictive model for PVST after splenectomy in patients with WD and hypersplenism was established, and the risk of postoperative PVST was predicted based on the perioperative color Doppler ultrasound and laboratory examination results, which could guide individualized treatment plans. The C-index in the modeling cohort and the internal validation in our study were 0.838 and 0.805, respectively, indicating that the model has good predictive accuracy. From the calibration curve and DCA curve, we can see that the model shows good consistency and clinical applicability.
Limitations
The study has some limitations. Firstly, the study was conducted at a single center with only a small number of patients, and thus the results need to be verified with multicenter data. Secondly, the independent risk factors in this study were all expressed as continuous numerical variables, which may not be sufficiently concise for clinical practice. To facilitate clinical application in the future, the optimal scale regression method could be considered for grouping the numerical variables and converting them into categorical variables. Third, although the stability of the nomogram of the prediction model was verified in this study by internal validation using the bootstrap method, no external validation was performed, and thus the generality of the findings is uncertain.
Conclusions
In conclusion, this study constructed a predictive model that included preoperative portal vein diameter, preoperative portal blood flow velocity, postoperative D-dimer, and PLT increase to predict PVST in patients with WD after splenectomy. The model was visualized in the form of a nomogram which demonstrated good accuracy in helping clinicians assess the risk of PVST early after splenectomy in patients with WD. The predictive model needs to be tested on a larger sample size with an external validation conducted to determine whether it can effectively predict the risk of PVST after splenectomy.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81774299, 82274493) and the Scientific Research Project of the Chinese Association of Ethnic Medicine (No. 2020ZY070-410033).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-826/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-826/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-826/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-826/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (No. 2022MCZQ18) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kahraman CY, Islek A, Tatar A, et al. A Novel Mutation of ATP7B Gene in a Case of Wilson Disease. Medicina (Kaunas) 2021;57:123. [Crossref] [PubMed]
- Sandahl TD, Laursen TL, Munk DE, et al. The Prevalence of Wilson's Disease: An Update. Hepatology 2020;71:722-32. [Crossref] [PubMed]
- Yang M, Liu J. Low-molecular weight heparin prevents portal vein system thrombosis after splenectomy: a systematic review and meta-analysis. ANZ J Surg 2020;90:2420-4. [Crossref] [PubMed]
- Huang L, Yu Q, Peng H. Hemorheological Alteration in Patients with Cirrhosis Clinically Diagnosed with Portal Vein System Thrombosis After Splenectomy. Med Sci Monit 2021;27:e931157. [Crossref] [PubMed]
- Sabbagh A, Keikhaei B, Joorabian M, et al. Retrospective study of the incidence of portal vein thrombosis after splenectomy in hematological disorders: Risk factors and clinical presentation. Blood Cells Mol Dis 2019;74:1-4. [Crossref] [PubMed]
- Yu-xing X, Xiao-ming W, Hui W, et al. Influencing factors of portal vein thrombosis after splenectomy in cirrhotic patients with Wilson’s disease. Journal of Hepatobiliary Surgery 2020;28:366-9.
- Yi-bing X, Qing-sheng Y, Jin-fang P, et al. High-risk Factors Analysis of Protal Vein System Thrombosis after Splenectomy in Hepatolenticular Degeneration and Hypersplenism. Clinical Journal of Traditional Chinese Medicine 2021;33:933-6.
- Inherited Metabolic Liver Disease Collaboration Group CSoH, Chinese Medical Association. Guidelines for the diagnosis and treatment of hepatolenticular degeneration (2022 edition). Zhonghua Gan Zang Bing Za Zhi 2022;30:9-20. [PubMed]
- Zheng Z, Yu Q, Peng H, et al. Research on Portal Venous Hemodynamics and Influencing Factors of Portal Vein System Thrombosis for Wilson's Disease after Splenectomy. Front Surg 2022;9:834466. [Crossref] [PubMed]
- Gerosa C, Fanni D, Congiu T, et al. Liver pathology in Wilson's disease: From copper overload to cirrhosis. J Inorg Biochem 2019;193:106-11. [Crossref] [PubMed]
- Cruz-Ramón V, Chinchilla-López P, Ramírez-Pérez O, et al. Thrombosis of the Portal Venous System in Cirrhotic vs. Non-Cirrhotic Patients. Ann Hepatol 2018;17:476-81. [Crossref] [PubMed]
- Xu W, Cheng Y, Tu B. Construction and validation of a nomogram for predicting the risk of portal vein thrombosis after splenectomy in patients with hepatitis B cirrhosis. Nan Fang Yi Ke Da Xue Xue Bao 2020;40:1265-72. [PubMed]
- Huang D, Tao M, Cao L, et al. Risk Factors and Anticoagulation Effects of Portal Vein System Thrombosis After Laparoscopic Splenectomy in Patients With or Without Cirrhosis. Surg Laparosc Endosc Percutan Tech 2019;29:498-502. [Crossref] [PubMed]
- O'Leary JG, Greenberg CS, Patton HM, et al. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019;157:34-43.e1. [Crossref] [PubMed]
- Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy. JHEP Rep 2021;3:100316. [Crossref] [PubMed]
- Senzolo M, Garcia-Tsao G, García-Pagán JC. Current knowledge and management of portal vein thrombosis in cirrhosis. J Hepatol 2021;75:442-53. [Crossref] [PubMed]
- Fujinaga A, Ohta M, Endo Y, et al. Clinical Significance of Splenic Vessels and Anatomical Features in Laparoscopic Splenectomy. J Laparoendosc Adv Surg Tech A 2021;31:632-7. [Crossref] [PubMed]
- Zhang Y, Xu BY, Wang XB, et al. Prevalence and Clinical Significance of Portal Vein Thrombosis in Patients With Cirrhosis and Acute Decompensation. Clinical Gastroenterology and Hepatology 2020;18:2564-72.e1. [Crossref] [PubMed]
- Swinson B, Waters PS, Webber L, et al. Portal vein thrombosis following elective laparoscopic splenectomy: incidence and analysis of risk factors. Surg Endosc 2022;36:3332-9. [Crossref] [PubMed]


