
Efficacy of treatments for pain and numbness in cancer survivors: a systematic review and meta-analysis
Introduction
The number of cancer survivors is steadily increasing due to advancements in cancer screening, treatment, and the aging of the population (1). The prevalence estimates for 2020 indicated that there were 50.6 million people alive worldwide who had been diagnosed with cancer in the previous 5 years (2). Some 64.8% of cancer survivors had reportedly lived with a diagnosis of cancer for 5 years or more, and 9.4% had lived this way for 25 years or more (3). Much focus has been placed on survival in cancer treatments, and the prevalence of pain or other symptoms, including numbness in survivors, has been underappreciated by both healthcare providers and patients. However, as cancer survivors live longer, cancer survivorship care has become increasingly important (4).
The prevalence rate of pain in patients during anticancer treatment is estimated to be 55%, and 66% for advanced, metastatic, and terminal diseases (5). Meanwhile, 39% of posttreatment cancer survivors reportedly experience pain, 28% of whom experience moderate-to-severe pain (5). Regarding numbness, 60% of patients with cancer and oxaliplatin-induced peripheral neuropathy report numbness and tingling 6–8 months after they complete treatment, and 20% show no improvement (6). Although paclitaxel-induced peripheral neuropathy improves in most patients within a few months after cessation of treatment, it can be a significant long-term problem in a subset of patients (6).
The experience of chronic pain and numbness often leads to psychological distress, limited activities of daily living (ADL), and poor quality of life (QoL) (7,8). A reason for the poor control of pain and numbness in cancer survivors may be the lack of education on effective symptom management among healthcare providers (7). Although pain in cancer survivors remains poorly studied and understood, it is mostly considered a consequence of cancer treatments including surgery, chemotherapy, hormone therapy, and radiation therapy (4). Any surgery can be a potential cause of chronic pain, and the incidence of persistent pain following breast surgery, thoracotomy, head and neck cancer surgery, and limb amputation is estimated to be 74%, 50%, 45%, and 64%, respectively (9-12). Chemotherapy-induced peripheral neuropathy (CIPN) is also a major cause of pain and numbness following cancer treatment which can lead to permanent symptoms and disability in up to 40% of cancer survivors (13). Aromatase inhibitor-induced arthralgia is a major cause of pain in breast cancer survivors, with an estimated prevalence of up to 50% (14). Since aromatase inhibitor therapy is often continued for up to 5 years, arthralgia may become a long-term problem for affected women. Additionally, radiation-induced brachial plexopathy may occur in up to 9% of women undergoing radiotherapy for breast cancer, and approximately 50% of cancer survivors have been reported to require opioids for the treatment of radiation-induced brachial plexopathy (4).
Opioid-based strategies for the treatment of active cancer pain have been validated; nevertheless, the use of chronic opioid treatment to control pain in cancer survivors remains controversial (15). Nonopioid pharmacotherapy, including duloxetine, is recommended for the treatment of CIPN in patients receiving active cancer treatment, but its efficacy for persistent pain and numbness in cancer survivors is unclear (6). Several randomized controlled trials (RCTs) have suggested that physical exercise, mindfulness, and acupuncture potentially improve pain in cancer survivors; however, no meta-analysis has validated or demonstrated the efficacy of these treatments for pain and numbness in cancer survivors. Therefore, this study aimed to identify current treatments and evaluate their efficacy for pain and numbness in cancer survivors. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-420/rc).
Methods
Data sources and search methods
The study was not registered, and a registered protocol was not prepared. Any materials such as data or analytic codes used in the study are not publicly available.
The PubMed, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Web of Science, PsycInfo, and CINAHL databases were electronically searched for articles published in English and Japanese languages from inception until 30 April 2022 using the following search term strategy: “cancer survivors” AND (“pain” OR “numbness” OR “tingling”) AND (“treatment” OR “management” OR “therapy” OR “strategy” OR “drug” OR “medication”).
Inclusion and study selection
Types of studies
RCTs published in English and Japanese languages that assessed treatments for pain or numbness initiated after the completion of active cancer treatment in cancer survivors were included for quantitative synthesis.
Types of participants
Cancer survivors with pain or numbness that were aged 18 years or older were included irrespective of tumor type, tumor stage, or type of anticancer treatment received. Cancer survivors were defined as those who met both of the following criteria: (I) patients diagnosed with cancer who had completed active cancer treatment, including surgery, chemotherapy, and radiation therapy; and (II) patients diagnosed with cancer whose conditions were stable or who had no evidence of recurrent or progressive disease.
Breast cancer survivors receiving hormone therapies were included if they had no recurrent or progressive disease. If it was not clear whether the cancer survivors met the inclusion criteria, the following studies were excluded: (I) those including more than 50% of patients with stage III or IV cancer, (II) those including more than 50% of patients receiving active cancer treatment, and (III) those including more than 50% of patients with terminal diseases.
Types of treatments
Treatments for pain and numbness were categorized into 7 types based on a previous meta-analysis: opioid therapy, nonopioid pharmacotherapy, interventional therapy, acupuncture, education/cognitive behavioral therapy (CBT), physical exercise, and alternative medicine (16).
Types of outcome measures
The primary outcome was the severity of posttreatment pain and numbness compared with that in controls, assessed by validated questionnaires, such as an 11-point numeric rating scale (NRS). Numbness included other expressions, such as tingling. If pain or numbness was measured at more than 1 site in the body, the score at the site of greatest interest was adopted.
Follow-up time
The follow-up time was set after the completion of the intervention. If the outcome was not measured upon the completion of the intervention, the follow-up time was set at the time of the first measurement of the outcome after its completion.
Statistical analysis
Selection of studies and assessment of search results
The retrieved abstracts were independently screened by 2 review authors (HA, RI) and the irrelevant articles were excluded. Subsequently, the full texts of all remaining studies were independently reviewed by 2 review authors (HA, RI) to ascertain whether they met the inclusion criteria. Disagreements were discussed between the reviewers, and if a resolution could not be reached, the final decision was made by a third reviewer (MS).
Data extraction and synthesis
Two review authors (MA, RT) independently extracted data that were relevant to study design, participant population, treatments, duration of intervention, follow-up time, measured outcomes, and results. For each outcome, posttreatment mean, posttreatment standard deviation (SD), and the sample size in each group were extracted. If posttreatment SD was not available, it was obtained from the posttreatment standard error, confidence interval (CI), t statistic, or P value (17). When only posttreatment median and interquartile range (IQR) were available, posttreatment mean and SD were estimated using Wan’s method (18,19). If none of these values were available, the missing SD was imputed from other studies in similar settings (17). If there was more than 1 SD candidate, the largest SD was adopted (17). If only graphs were available, values were obtained from the graphs. When there were multiple control groups or intervention groups, the results were combined into a single control or intervention group using the method described in the Cochrane handbook for systematic reviews of interventions (17).
Review Manager (RevMan) 5.4 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used to estimate the standardized mean difference (SMD) using a random-effects model due to the different scoring systems employed for measuring pain and numbness, and the results were formatted into forest plots. Heterogeneity among the included studies was assessed using forest plots and I2 tests, with I2 values of >50% indicating substantial heterogeneity (20).
Subgroup analysis
Subgroup analyses were conducted by similar treatment clusters in each treatment group.
Missing data
If the required data were not available, attempts were made to contact the corresponding authors via email to obtain the missing data.
Bias assessment
The risk of bias for studies were independently assessed by 2 review authors (MA, RT) by evaluating random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases; the judgments were assigned as a high, low, or unknown risk for each item (21). A funnel plot was prepared to evaluate the publication bias and was visually examined for signs of asymmetry.
Quality of evidence
The quality of evidence was independently assessed by 2 review authors (MA, RT). The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines were used for assessing the quality of evidence of each outcome, categorized as high, moderate, low, and very low (22).
Results
Literature search
The systematic search identified a total of 1,252 articles in English (Figure 1). No Japanese articles were identified. Following the removal of duplicate articles, 708 articles remained, of which 581 were excluded after a reading of the titles and abstracts; 91 were excluded after a reading of the full text due to wrong study design, population, or outcome. Finally, 36 RCTs (2,870 cancer survivors) were included in the meta-analysis (Table 1).
Table 1
| Author, year, country | Participants | Age (years), N, adherence to protocol | Intervention | Comparator | Duration/follow-up time | Outcome measures |
|---|---|---|---|---|---|---|
| Ellison, 1997, United States (23) | Cancer survivors with postsurgical neuropathic pain | 66 (IG), 64 (CG)†, N=99, 71.7% | A 0.075% capsaicin cream | Placebo cream | 8 weeks/week 8 | Improvements in pain (VAS) |
| Gewandter, 2014, United States (24) | Cancer survivors with CIPN | Not clear, N=458, 80.6% | Ketamine plus amitriptyline cream twice per day | Placebo cream | 6 weeks/week 6 | No observed changes in pain (NRS), numbness, or tingling (NRS) |
| Shapiro, 2016, United States (25) | Breast cancer survivors with aromatase inhibitor-associated musculoskeletal symptom | 60.9±8.8, N=113, 97.3% | Vitamin D3 at 4,000 IU/day | Vitamin D3 at 600 IU/day | 24 weeks/week 24 | No observed change in pain (WOMAC) |
| Peng, 2018, China (26) | Breast cancer survivors with aromatase inhibitor–associated musculoskeletal symptoms | 57.3±6.4 (IG), 59.8±8.0 (CG), N=84, 91.7% | Chinese medicine Yi Shen Jian Gu | Placebo | 12 weeks/week 12 | Improvements in pain (BPI-SF) and musculoskeletal symptoms |
| De Groef, 2018a, Belgium (27) | Breast cancer survivors with persistent upper limb pain | 53.4±10.0 (IG), 56.6±10.0 (CG), N=50, 96.0% | A single botulinum toxin A infiltration and 3 months of physical therapy | Placebo infiltration and 3 months of physical therapy | 12 weeks/week 12 | No observed change in upper limb pain (VAS) |
| Kim, 2017, Korea (28) | Breast cancer survivors with dyspareunia | 48.1±6.3 (IG), 49.2±5.2 (CG), N=136, 78.7% | pH-balanced vaginal gel | Placebo gel | 8 weeks/week 8 | No observed change in pain (FSFI) and overall sexual function |
| Mao, 2021, United States (29) | Cancer survivors with chronic musculoskeletal pain | 62.1±12.7, N=358, 95.0% | 10 sessions of electroacupuncture or auricular acupuncture over 10 weeks | Usual care | 12 weeks/week 12 | Improvements in pain (BPI) |
| Crew, 2007, United States (30) | Breast cancer survivors with aromatase inhibitor-induced joint symptoms | 59 [46–73]†, N=21, 90.5% | Twice weekly, full body and auricular acupuncture | Usual care | 6 weeks/week 6 | Improvement in pain (BPI-SF), function, and physical well-being |
| Lu, 2020, United States (31) | Breast cancer survivors with CIPN | 54 [32–68] (IG), 53.5 [26–71] (CG)†, N=40, 77.5% | 18 acupuncture treatments in upper and lower limbs over 8 weeks | Usual care | 8 weeks/week 8 | Improvements in pain (BPI-SF) and numbness (PNQ, FACT-NTX) |
| Burch, 2020, United States (32) | Cancer survivors with symptoms including pain | 60±3 (IG), 59±2 (CG), N=38, 89.5% | 4–6 times weekly, heart rate variability biofeedback training | Usual care | Not clear/week 1 after training | No observed change in pain (BPI) or sleep-related symptom |
| Prinsloo, 2017, United States (33) | Cancer survivors with CIPN-related pain | 62.5±10.3, N=71, 87.3% | At least twice weekly, 20 sessions of electroencephalogram neurofeedback | Usual care | 10 weeks/week 10 | Improvements in pain (BPI-SF), numbness, and tingling (PQAS) |
| Alberts, 2020, United States (34) | Adult survivors of childhood cancer with chronic pain | 44.1±8.7, N=65, 96.9% | Breathing exercise using wearable respiratory monitoring and feedback | Psychoeducation | 4 weeks/week 4 | No observed changes in pain (BPI) and quality of life |
| Kelleher, 2021, United States (35) | Colorectal cancer survivors with pain and psychological distress | 59.5±10.5, N=31, 93.5% | Five sessions of telephone-based coping skills training | Usual care | 7 weeks/week 7 | No observed change in pain (BPI) |
| Smith, 2019, United States (36) | Breast cancer survivors with chronic pain | 56.7±8.7, N=122, 73.0% | Online symptom self-management curriculum for 18 weeks | Usual care | 18 weeks/week 18 | No observed changes in pain (BPI) and self-efficacy, and improvement in depression |
| Lengacher, 2016, United States (37) | Breast cancer survivors with symptoms including pain | 56.6±9.7, N=322, 92.9% | Mindfulness-based stress reduction program for 6 weeks | Usual care | 6 weeks/week 6 | No observed changes in pain (BPI) and general health |
| Shergill, 2022, Canada (38) | Breast cancer survivors with chronic neuropathic pain | 51.3±11.4 (IG), 55.1±9.6 (CG), N=98, 75.5% | Mindfulness-based stress reduction program for 12 weeks | Usual care | 12 weeks/week 12 | No observed change in pain (BPI) |
| Bao, 2020, United States (39) | Breast and gynecological cancer survivors with persistent CIPN | 61.7 [35–79]*, N=41, 87.8% | Daily yoga intervention for 8 weeks | Usual care | 8 weeks/week 8 | Improvement in pain (NRS) and no observed change in numbness and tingling (NRS) |
| Tsai, 2021, China (40) | Breast cancer survivors with aromatase inhibitor‑associated knee joint pain | 53.9±6.4, N=60, 86.7% | Twice-weekly yoga intervention for 6 weeks | Massage intervention | 6 weeks/week 7 | No observed change in pain (WOMAC) |
| Cantarero-Villanueva, 2012, Spain (41) | Postsurgical breast cancer survivors with neck and shoulder pain | 48±8 (IG), 47±9 (CG), N=66, 98.5% | Thrice weekly water-based exercise training over 8 weeks | Usual care | 8 weeks/week 8 | Improvement in neck and shoulder-axillary pain (VAS) |
| Cantarero-Villanueva, 2017, Spain (42) | Colon cancer survivors with chronic pain | 57.5±8.1 (IG), 62.3±7.9 (CG), N=46, 87.0% | Thrice weekly; lumbopelvic stabilization, aerobic, and stretching exercise over 8 weeks | Usual care | 8 weeks/week 8 | No observed change in pain (BPI) and improvement in lumbopelvic musculoskeletal conditions |
| Fields, 2016, United Kingdom (43) | Breast cancer survivors with aromatase inhibitor-associated arthralgia | 63±8, N=40, 100.0% | Once weekly, supervised group Nordic walking training for 6 weeks | Usual care | 12 weeks/week 12 | No observed change in pain (BPI-SF) |
| Fu, 2022, United States (44) | Breast cancer survivors with chronic pain and symptoms related to lymphedema | 56.7±10.6, N=120, 95.0% | Web- and mobile-based self-care, daily lymphatic exercises program | Web- and mobile-based arm precaution program | 12 weeks/week 12 | Improvements in pain (Likert scale), arm/hand swelling, and shoulder/arm movement |
| Nyrop, 2017, United States (45) | Breast cancer survivors with aromatase inhibitor-associated arthralgia | 63.8±8.3, N=62, 85.5% | Walking exercise for at least 150 min per week for 6 weeks | Usual care | 6 weeks/week 6 | No observed change in pain (VAS) and improvement in stiffness and activities of daily living |
| Baglia, 2019, United States (46) | Breast cancer survivors with aromatase inhibitor-induced arthralgia | 61.2±7.0, N=121, 88.4% | Twice weekly, strength training and aerobic exercise over 12 months | Usual care | 48 weeks/week 48 | Improvements in pain (SF-36) and quality of life |
| Fernández-Lao, 2012, Spain (47) | Postsurgical breast cancer survivors with neck and shoulder pain | 49±8 (IG), 47±8 (CG), N=44, 97.7% | Thrice weekly, individual multidimensional physical therapy for 8 weeks | Usual care | 8 weeks/week 8 | Improvement in neck and shoulder/axillary pain (VAS) and reduction in pressure pain thresholds |
| Galiano-Castillo, 2016, Spain (48) | Breast cancer survivors with symptoms including pain | 47.4±9.6 (IG), 49.2±7.9 (CG), N=76, 94.7% | Thrice weekly, internet-based, tailored multidimensional exercise program | Usual care | 8 weeks/week 8 | Improvements in pain (BPI), quality of life, and muscle strength |
| Irwin, 2015, United States (49) | Breast cancer survivors with aromatase inhibitor-induced arthralgia | 62.0±7.0 (IG), 60.5±7.0 (CG), N=121, 68.6% | Twice weekly, supervised resistance training and a home-based aerobic exercise | Usual care | 48 weeks/week 48 | Improvement in pain (BPI) |
| Paulo, 2019, Brazil (50) | Older breast cancer survivors undergoing aromatase inhibitor therapy | 63.2±7.1 (IG), 66.6±9.6 (CG), N=36, 88.9% | Thrice weekly, combined resistance plus aerobic training for 9 months | Usual care | 36 weeks/week 36 | Improvements in pain (SF-36) and quality of life |
| McNeely, 2008, Canada (51) | Postsurgical head and neck cancer survivors with shoulder dysfunction | 52 (32–76)†, N=52, 92.3% | Twice weekly, supervised progressive resistance exercise training | Standardized therapeutic exercise | 12 weeks/week 12 | Improvements in shoulder pain (SPADI) and shoulder disability |
| Castro-Martín, 2017, Spain (52) | Breast cancer survivors with cervical and shoulder pain | 50.1±8.8, N=21, 100.0% | Two sessions of myofascial induction that focused on the upper limb area | Placebo electrotherapy | 1 day/day 1 | Improvements in pain in the affected arm (VAS), cervical/shoulder range of motion, and no observed change in anxiety |
| De Groef, 2018b, Belgium (53) | Breast cancer survivors with persistent arm pain | 55.3±7.5 (IG), 53.1±7.5 (CG), N=50, 96.0% | Once weekly individual myofascial therapy | Once weekly placebo therapy | 12 weeks/week 12 | Improvement in pain (VAS) |
| Rangon, 2018, Brazil (54) | Breast cancer survivors with chronic myofascial pain in the upper trunk region | 54.9±7.1, N=20, 90.0% | Twice weekly, 10 sessions of ischemic compression plus kinesiotherapy | Twice weekly, 10 sessions of kinesiotherapy | 5 weeks/week 5 | Improvements in pain (NRS) and pressure pain threshold |
| Serra-Añó, 2019, Spain (55) | Breast cancer survivors with postoperative persistent pain | 53.7±9.1, N=24, 100.0% | Myofascial release treatment over 4 weeks | Placebo manual lymphatic drainage treatment | 4 weeks/week 4 | Improvements in pain (VAS), range of motion, and quality of life |
| Conejo, 2018, Spain (56) | Breast cancer survivors with aromatase inhibitor-associated musculoskeletal disorders | 66.3 [50–82]*, N=40, 90.0% | Neuromuscular taping on upper limbs, cervical and lumbar site | Sham neuromuscular taping | 5 weeks/week 5 | Improvements in pain (VAS), quality of life, and fatigue |
| Eaton, 2021, United States (57) | Cancer survivors with chronic pain | 52.7±13.1, N=40, 75.0% | Listening to a hypnosis recording daily for 4 weeks | Usual care | 4 weeks/week 4 | No observed change in pain (NRS) |
| Bao, 2021, United States (58) | Solid tumor survivors with persistent CIPN | 60 (51–79) (IG), 62 (43–86) (CG)†, N=51, 90.2% | 10 acupuncture treatments over 8 weeks | Sham acupuncture | 8 weeks/week 8 | No observed change in numbness (FACT/GOG-NTX) |
Age is presented as mean ± SD, median (range) (†), or mean (range) (*). IG, intervention group; CG, control group; VAS, visual analogue scale; CIPN, chemotherapy-induced peripheral neuropathy; NRS, numeric rating scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis; BPI, Brief Pain Inventory; FSFI, Female Sexual Function Index; PNQ, Patient Neurotoxicity Questionnaire; FACT/GOG-NTX, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity; PQAS, Pain Quality Assessment Scale; SF-36, Medical Outcomes Study 36-item Short-Form Health Survey; SPADI, Shoulder Pain and Disability Index; SD, standard deviation.
Participants and study characteristics
Participants were recruited from various settings, such as university hospitals, breast cancer centers, clinics, rehabilitation centers, and internet cancer support groups. The studies were conducted in Belgium, Brazil, Canada, China, Korea, Spain, the United Kingdom, and the United States. Participants involved in the studies were 18–86 years of age. Overall adherence to intervention protocols was high (87.5%; range, 68.6–100%) (Table 1). A total of 24 studies investigated breast cancer survivors only, among which 10 investigated breast cancer survivors with aromatase inhibitor-associated arthralgia. There were 2 studies that involved colon cancer survivors and 1 that involved head and neck cancer survivors. The remaining 9 studies included multiple types of cancer. Although the cancer survivors included in most studies completed active cancer treatment and had no recurrent or progressive disease, some studies might have included patients receiving active cancer treatment or with progressive disease.
Treatments
The included treatments comprised nonopioid pharmacotherapy (topical cream, oral medication), acupuncture, education/CBT (biofeedback, coping skills training), physical exercise (stretching exercise, aerobic exercise, resistance training), and alternative medicine (myofascial therapy). No study focused on widely used nonopioid pharmacological therapies (e.g., acetaminophen, nonsteroidal anti-inflammatory drugs, anticonvulsants, antidepressants), opioid therapy, or interventional therapy (e.g., nerve block, spinal cord stimulation). The duration of the intervention ranged from 1 day to 48 weeks, and the median duration of intervention was 8 weeks (IQR, 6–12). The median follow-up time was 8 weeks (IQR, 6–12).
Outcome measures
Among 36 RCTs, 35 studies (n=2,813) measured pain and 5 (n=566) measured numbness. Pain and numbness symptom outcomes were measured using validated questionnaires, such as the NRS, visual analogue scale (VAS), Brief Pain Inventory (BPI), Medical Outcomes Study 36-item Short-Form Health Survey (SF-36), the Shoulder Pain and Disability Index (SPADI), Female Sexual Function Index (FSFI), Western Ontario and McMaster Universities Osteoarthritis (WOMAC), Functional Assessment of Cancer Therapy-Neurotoxicity (FACT-NTX), Patient Neurotoxicity Questionnaire (PNQ), or Pain Quality Assessment Scale (PQAS) (Table 1).
Missing data
For the study by Ellison et al. (23), we contacted the author via e-mail to obtain posttreatment means and SDs or raw data, but we were unable to obtain the required information.
Risk-of-bias assessment
The results of risk-of-bias assessment are presented in Figure 2. Overall, 29 studies were at high risk of bias because the participants and providers were not blinded to treatment assignment and the outcomes were self-assessed.
Quality of evidence
Quality of evidence was rated moderate in the meta-analysis regarding the effect of physical exercise on pain, low for the effect of acupuncture on pain, and very low for that of other treatments on pain and numbness. Downgrading of evidence ratings in meta-analyses was mainly due to the limited number of participants, risk of bias arising from blinding and self-assessment of outcomes, and heterogeneity between studies.
Publication bias
The funnel plot for pain measurement appeared to be approximately symmetrically distributed, indicating no clear publication bias (Figure 3A). Publication bias for numbness could not be determined due to the limited number of studies (Figure 3B).

Adverse events
No severe complications were reported in 36 studies, and the treatments were considered well tolerated.
Meta-analysis for pain
A total of 35 RCTs investigated the effectiveness of treatment on pain among an accumulated 2,813 cancer survivors. The causes of pain in the included studies were aromatase inhibitor-associated arthralgia in breast cancer survivors in 10 studies, persistent postmastectomy pain in the neck, shoulder, and upper limb in 8 studies, CIPN in 4 studies, postsurgical neuropathic pain in 2 studies, dyspareunia in 1 study, shoulder pain in postsurgical head and neck cancer survivors in 1 study, and nonspecific pain in 9 studies. Most included studies did not clarify the medicines that cancer survivors were using before participation. Studies investigating the effectiveness of opioid therapy and interventional therapy were not identified. The efficacy of nonopioid pharmacotherapy was investigated in 6 studies, acupuncture in 3, education/CBT in 7, physical exercise in 13, and alternative medicine in 6 studies.
Effect of nonopioid pharmacotherapy on pain
The effectiveness of nonopioid pharmacotherapy on pain was investigated in 6 RCTs (n=782) (23-28). Treatments included capsaicin cream (23) and ketamine plus amitriptyline cream (24) for neuropathic pain, vitamin D3 (25) and the Chinese medicinal formula Yi Shen Jian Gu (26) for aromatase inhibitor-associated musculoskeletal pain, botulinum toxin A for upper limb pain (27), and pH-balanced vaginal gel for dyspareunia (28). There were 4 studies that included breast cancer survivors, and the remaining 2 studies included survivors of various types of cancer. The control groups received either placebo treatment or a low-dose treatment. Figure 4 shows the results of the meta-analysis for nonopioid pharmacotherapy. In this meta-analysis, the missing posttreatment SD values in the study by Ellison et al. (23) were imputed with the posttreatment SD values in the study by Gewandter et al. (24) because of the similarity in study settings (17). No significant difference in improvement in pain was shown between the nonopioid pharmacotherapy groups and the control groups [SMD −0.19; 95% confidence interval (CI): −0.46 to 0.08; P=0.16]. Heterogeneity between studies was substantial (I2=65%; P=0.01), and the quality of evidence was rated very low.
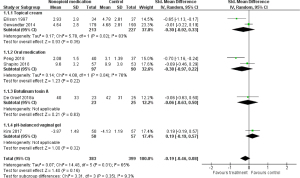
We performed a subgroup analysis of topical cream treatment [2 studies; n=440; capsaicin cream (23), ketamine plus amitriptyline cream (24)] for neuropathic pain (Figure 4), and no significant effect was shown (SMD −0.30; 95% CI: −0.92 to 0.33; P=0.35). Subgroup analysis of oral medication [2 studies; n=187; vitamin D3 (25), the Chinese medicine Yi Shen Jian Gu (26)] for aromatase inhibitor-associated musculoskeletal pain (Figure 4) also showed no significant effect (SMD −0.38; 95% CI: −0.97 to 0.22; P=0.22). Subgroup analysis for botulinum toxin A and vaginal gel on pain involved only 1 study each (Figure 4). The effect of botulinum toxin A on upper limb pain was investigated in a placebo-controlled trial that did not show a significant effect (27). No significant effect of the pH-balanced vaginal gel on dyspareunia was demonstrated compared with a placebo gel (28).
Effect of acupuncture on pain
A total of 3 RCTs (n=409) were included in the meta-analysis for acupuncture, including electroacupuncture, auricular acupuncture, and traditional acupuncture (29-31); 2 of these studies included breast cancer survivors and 1 included survivors of various types of cancer. The control groups were placed on the waiting list or received usual care. Mao et al. (29) reported the results of electroacupuncture and auricular acupuncture separately; thus, the results of the 2 groups were combined into a single intervention group using the method in the Cochrane handbook for systematic reviews of interventions (17). The result of the meta-analysis is shown in Figure 5. Across the studies, the efficacy of acupuncture on pain was significant, and the overall effect size was large (SMD −0.80; 95% CI: −1.04 to −0.56; P<0.00001). Between-study heterogeneity in the effects of treatments was low (I2=0%, P=0.47), and the quality of evidence was rated low.
Effect of education/CBT on pain
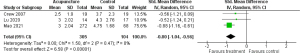
The meta-analysis for the effect of education/CBT on pain included 7 RCTs (n=655) (32-38). Of these, 3 studies focused on biofeedback (32-34); 2 on coping skills training (35,36), and 2 on mindfulness (37,38). There were 3 studies that included breast cancer survivors and 1 that included colon cancer survivors, with the remaining 3 studies including survivors of various types of cancer. The control groups were placed on the waiting list or received usual care or minimal intervention. Figure 6 displays the results of the meta-analysis for education/CBT. Significant pain reduction was not found in the education/CBT group in comparison with that in the control group (SMD −0.15; 95% CI: −0.45 to 0.15; P=0.33). The heterogeneity between studies was substantial (I2=65%; P=0.008) and the quality of evidence was rated very low.

Subgroup analysis for biofeedback (3 studies; n=159) included heart rate variability biofeedback (32), electroencephalogram neurofeedback (33), and respiratory biofeedback using a wearable device (34), with results indicating a significant effect on pain and a medium effect size (SMD −0.54; 95% CI: −0.92 to −0.17; P=0.004; Figure 6). Subgroup analysis for coping skills training [2 studies; n=119; telephone-based coping skills training (35), online symptom self-management training (36)] did not show a significant effect on pain (SMD 0.11; 95% CI: −0.45 to 0.67; P=0.69; Figure 6). Subgroup analysis for mindfulness-based stress reduction training on pain (2 studies; n=377) (37,38) showed no significant effect compared with usual care (SMD 0.11; 95% CI: −0.09 to 0.31; P=0.28; Figure 6).
Effect of physical exercise on pain
The efficacy of physical exercise on pain was investigated in 13 RCTs (n=761) (39-51). Treatments included a variety of exercise types, durations, and frequencies. A total of 10 studies focused on breast cancer survivors, 1 on colon cancer survivors, 1 on head and neck cancer survivors, and 1 on breast and gynecological cancer survivors. Of the 10 studies focusing on breast cancer survivors, 6 included patients with aromatase inhibitor-associated arthralgia. The control groups were placed on the waiting list or received usual care or minimal intervention, such as conventional therapeutic exercise. The results of the meta-analysis of 13 studies are shown in Figure 7. Physical exercise significantly reduced pain in cancer survivors, and the effect size of the treatments was large (SMD −0.84; 95% CI: −1.14 to −0.55; P<0.00001). Heterogeneity between studies was substantial (I2=72%; P<0.0001), and the quality of evidence was rated moderate.
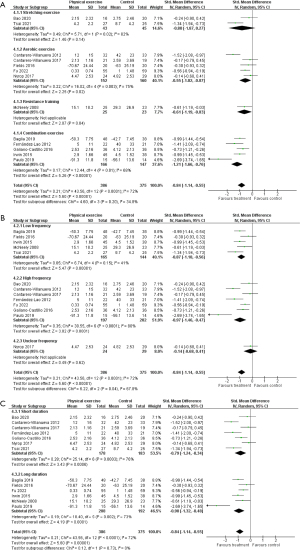
In general, the types of physical exercise are classified as stretching exercise, aerobic exercise, and resistance training (59). We performed subgroup analyses on exercise type for stretching exercise, aerobic exercise (plus stretching exercise), resistance training (plus stretching exercise), and a combination of aerobic exercise and resistance training (plus stretching exercise). The subgroup analysis for stretching exercise (2 studies; n=88) included yoga (39,40) and did not show a significant effect on pain (SMD −0.80; 95% CI: −1.87 to 0.27; P=0.14; Figure 7A). Subgroup analysis for aerobic exercise (plus stretching exercise) involved water-based physical exercise, core stabilization with aerobic exercise, Nordic walking, web- and mobile-based lymphatic exercise, and home-based walking exercise (5 studies; n=312) (41-45), and demonstrated a significant effect on pain (SMD −0.55; 95% CI: −1.02 to −0.07; P=0.02; Figure 7A). In the subgroup analysis for combination exercise (5 studies; n=313) (46-50) the result showed a significant effect on pain (SMD −1.21; 95% CI: −1.66 to −0.76; P<0.00001; Figure 7A). Only 1 study investigated the effect of resistance training (51), and it showed a significant effect (Figure 7A).
Exercise frequencies in the included studies were daily in 2 studies, thrice weekly in 5 studies, twice weekly in 4 studies, and once weekly in 1 study (Table 1). In the subgroup analysis regarding exercise frequency, both low-frequency exercise (twice weekly or less, 5 studies; n=309; SMD −0.87; 95% CI: −1.18 to −0.56; P<0.00001) (40,43,46,49,51) and high-frequency exercise (thrice weekly or more, 7 studies; n=399; SMD −0.97; 95% CI: −1.46 to −0.47; P<0.0001) (39,41,42,44,47,48,50) showed significant effects on pain (Figure 7B).
The duration of the intervention in the included studies ranged from 6 to 48 weeks, and the median duration was 8 weeks (IQR, 8–12; Table 1). In the subgroup analysis regarding the duration of exercise intervention, both short duration exercise (8 weeks or less, 7 studies; n=361; SMD −0.79; 95% CI: −1.24 to −0.34; P=0.0006) (39-42,45,47,48) and long duration exercise (more than 8 weeks; 6 studies; n=400; SMD −0.90; 95% CI: −1.32 to −0.48; P<0.0001) (43,44,46,49-51) showed a significant effect on pain (Figure 7C).
Effect of alternative medicine on pain
Meta-analysis for the effect of alternative medicine on pain included 6 RCTs (n=206) (52-57). There were 4 studies that focused on myofascial therapy for breast cancer survivors with musculoskeletal pain (52-55), 1 on neuromuscular taping for breast cancer survivors with aromatase inhibitor-associated arthralgia (56), and 1 on hypnosis for survivors of various types of cancer with chronic pain (57). The control groups were placed on the waiting list or received placebo therapy, usual care, or conventional therapy. Figure 8 demonstrates the results of the meta-analysis. Across the studies, significant pain reduction was shown in the alternative medicine group compared with that in the control group, and the overall effect size of treatments was medium (SMD −0.44; 95% CI: −0.71 to −0.16; P=0.002). Low heterogeneity was shown between studies (I2=0%; P=0.67), and the quality of evidence was rated very low.

The subgroup analysis for myofascial therapy included 4 studies (n=132) (52-55) and showed a significant effect on pain in breast cancer survivors with musculoskeletal pain (SMD −0.38; 95% CI: −0.72 to −0.03; P=0.03; Figure 8). Subgroup analysis for neuromuscular taping and hypnosis included 1 study each. The study on neuromuscular taping (56) showed a significant effect on pain in breast cancer survivors with musculoskeletal pain (Figure 8). The study investigating hypnosis (57) did not show a significant effect on chronic pain in cancer survivors (Figure 8).
Meta-analysis for numbness
A total of 5 RCTs investigated the effectiveness of treatment on numbness in 566 cancer survivors (24,31,33,39,58). The causative disease of numbness was CIPN in all studies. Treatments included nonopioid pharmacotherapy, acupuncture, education/CBT, and physical exercise.
The meta-analysis for acupuncture included 2 studies (n=99) (31,58). Bao et al. (58) reported the results of sham acupuncture and usual care separately; thus, the results of the 2 groups were combined into a single control group using the method in the Cochrane handbook for systematic reviews of interventions (17). Across the studies, no significant effect of acupuncture on numbness was shown (SMD −0.81; 95% CI: −1.65 to 0.02; P=0.06; Figure 9A). Between-study heterogeneity was substantial (I2=69%; P=0.07), and the quality of evidence was rated very low.

In the meta-analysis for the effect of nonopioid pharmacotherapy, education/CBT, and physical exercise on numbness, only 1 study each was included, and the quality of evidence was rated very low. Education/CBT (neurofeedback) (33) demonstrated a significant effect on numbness compared with usual care (Figure 9B). The effect of nonopioid pharmacotherapy (ketamine plus amitriptyline cream) (24) and physical exercise (yoga) (39) did not show significant effects on numbness compared with placebo therapy and usual care, respectively (Figure 9C,9D).
Discussion
To the best of our knowledge, this systematic review and meta-analysis is the first to evaluate the effects of current treatments on pain and numbness in cancer survivors. Our results suggested that several types of treatment could significantly reduce pain. The overall effect size of treatments was large for the effect of physical exercise and acupuncture on pain and medium for that of alternative medicine on pain. With respect to cancer type, 24 of the 36 included studies investigated only breast cancer survivors, and 10 of the 24 studies targeted only breast cancer survivors with aromatase inhibitor-associated arthralgia. The reasons why many studies have focused on breast cancer survivors may be as follows: (I) breast cancer survivors have more trouble with pain and numbness than do survivors of other cancer types, (II) stable patients are more common among survivors of breast cancer than among those with other cancer types, and (III) recruiting a homogeneous patient population of breast cancer survivors may be easier than it would be for survivors of other cancer types. If the ease of recruitment of breast cancer survivors is the major cause of a biased number of studies, such bias would be of great concern because other cancer survivors might have been underrepresented.
Effect of treatment on pain
The prevalence of pain is higher in cancer survivors than in the general population, as is the rate of prescription opioid use (60,61). Compared to those without cancer, cancer patients anecdotally have a reduced risk of nonmedical opioid use and opioid-related death; nonetheless, a previous study reported that rates of nonmedical opioid use were similar between cancer survivors and the general population (61). Severe cases of nonmedical opioid use may lead to death; therefore, a meta-analysis of the effects and adverse events of opioid therapy on pain in cancer survivors is considered important. However, no RCTs on opioid therapy for cancer survivors have been conducted, despite many cancer survivors receiving opioid therapy. Usually, opioids are prescribed only during the therapeutic phase of cancer. Initiating opioid therapy after the completion of cancer treatment may lead to opioid use disorder and nonmedical opioid use. Therefore, planning such an RCT may have inherent ethical issues. In the present meta-analysis, no evidence was found for or against opioid use as a treatment for pain and numbness in cancer survivors. However, some guidelines have recommended the use of opioids in carefully selected cancer survivors with chronic pain who do not respond to conservative management; hence, opioid administration to cancer survivors may be desirable, when necessary (62,63).
As for nonopioid pharmacotherapy, the relevant treatments in the 6 studies included in this meta-analysis are not widely used in clinical settings. Acetaminophen, nonsteroidal anti-inflammatory drugs, anticonvulsants, and antidepressants are widely used for pain in cancer patients. However, studies investigating the efficacy of these widely used nonopioid analgesics on pain in cancer survivors were not identified. The reason for the lack of such studies targeting cancer survivors may be that most analgesics, similarly to opioids, are initiated during the treatment phase of cancer but are not initiated after completion of cancer treatment.
Our meta-analysis suggested that physical exercise could significantly reduce pain in cancer survivors, with a large effect size. Mishra et al. (64) performed a meta-analysis on physical exercise in cancer survivors. However, their meta-analysis focused on health-related QoL, and definitive conclusions were not drawn regarding the effect on pain. A few other meta-analyses assessed the effect of exercise on pain in breast cancer survivors with aromatase inhibitor-associated arthralgia (65-67). The included studies were not the same due to a difference in inclusion criteria, and the results were controversial. Regarding noncancer pain, the efficacy of physical exercise has been demonstrated in meta-analyses (68-70). Moreover, previous RCTs on physical exercise have shown improvements in function, ADL, or QoL in patients with chronic noncancer pain (71-73). Although this meta-analysis did not investigate outcomes other than pain and numbness, physical exercise may be more beneficial than other treatments, considering its various benefits aside from pain control in patients with chronic noncancer pain. The effect of optimal type, frequency, and duration of physical exercise on chronic noncancer pain is controversial (69,74,75). Our meta-analysis suggested that a combination, higher frequency, and longer duration of exercise may be more effective for reducing pain in cancer survivors. The results could provide some direction for future research, although the quality of evidence of these subgroup analyses was low or very low.
The effect of biofeedback on chronic noncancer pain was demonstrated with medium-to-large effect sizes in previous meta-analyses (76,77). Meta-analyses for mindfulness (78) and coping skills training (79) also showed a significant effect on chronic noncancer pain, but the effect sizes were small. The present meta-analysis showed a significant effect of biofeedback on pain in cancer survivors, although coping skills training and mindfulness did not show significant effects. These interventions, however, had almost no side effects and would be tolerable even in cancer survivors with difficulty in exercising. Furthermore, additive or synergistic effects may result when these interventions are combined with physical exercise.
A previous meta-analysis assessed the effect of acupuncture on nonspecific low-back pain and reported that acupuncture showed a significant effect on pain compared with no treatment, in contrast to sham acupuncture, which showed no significant effect (80). In the present meta-analysis, acupuncture showed a medium-to-large effect on pain in cancer survivors; however, the results should be interpreted with caution in the context of the high placebo effect of acupuncture and limitations related to difficulty in blinding the procedure. A previous study reported the significant effect of phantom acupuncture on pain (81). All 3 studies included in this meta-analysis did not use sham acupuncture as a control; therefore, a clear conclusion could not be drawn without a comparison with phantom acupuncture.
With respect to interventional therapy, some previous systematic reviews investigated the effects of radiofrequency thermocoagulation on pain in cancer patients (82,83). A study investigated the effect of radiofrequency thermoablation and radiotherapy combined treatment for bone metastasis in cancer patients (82). Another study assessed the effect of stereotactic radiofrequency thalamotomy on intractable cancer pain (83). Both studies demonstrated significant effects on pain; however, these studies focused on cancer patients, not cancer survivors, and included no RCTs. Due to the treatment’s invasiveness and difficulty in blinding, conducting RCTs on interventional therapy would be difficult.
In the meta-analysis for the effect of alternative medicine on pain, a subgroup analysis for myofascial therapy demonstrated a significant effect on musculoskeletal pain in breast cancer survivors. Pinheiro da Silva et al. (84) performed a meta-analysis on the effect of manual therapy, including myofascial therapy for breast cancer survivors, and demonstrated a small effect on chronic musculoskeletal pain. Regarding meta-analysis for myofascial therapy on noncancer pain, Ughreja et al. (85) found a large effect in patients with fibromyalgia, yet Chen et al. (86) did not find a significant effect in patients with low-back pain. Although generalization of the result is not possible, myofascial therapy may be effective for chronic musculoskeletal pain in breast cancer survivors, in combination with other treatments, including physical exercise and education/CBT.
Effect of treatment on numbness
Only 5 studies investigating the effect of treatments on numbness were identified in this meta-analysis. Although the meta-analysis for acupuncture included 2 studies, that for other treatments included only 1 study, and therefore the quality of evidence for each was rated very low. The efficacy of gabapentinoids or duloxetine on numbness in cancer patients has been demonstrated in some studies; however, these studies were not included in the present meta-analysis because the participants were either undergoing active cancer treatment or had advanced disease, and were not eligible for inclusion (87-89). The absence of such studies focusing on cancer survivors may be because most medications are initiated during the treatment phase of cancer and not after the completion of cancer treatment, as is the case with opioid analgesics for pain in cancer survivors. A previous study reported that the median duration of paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer was 2 years, and paclitaxel-induced peripheral neuropathy persisted in 64% and 41% of patients at 1 year and 3 years after initiation, respectively (90). Numbness and tingling, characteristic symptoms of CIPN, were reported to be closely related to an impairment in fine motor skills and ADL, as well as a reduction in QoL that was clinically relevant (8,91). The lack of studies on treatment for numbness in cancer survivors is a matter of concern. Our study suggests that the efficacy of treatments for pain and numbness is not necessarily the same. Hence, there is a need for studies evaluating treatments for numbness separately from pain in cancer survivors.
Limitations
This study had several limitations. First, the lack of a registered protocol was a limitation. Second, the effects of opioid therapy and interventional therapy for pain and numbness as well as those of alternative medicine for numbness could not be determined due to the lack of studies. In addition, the number of participants was too small to determine the precise treatment effects of alternative medicine for pain and all the treatments for numbness. Third, the heterogeneity in each treatment was too large to determine its effectiveness. Fourth, the proportion of patients receiving active cancer treatment or having progressive disease was not clear in some studies. Fifth, there might have been some problems in classifying the type of treatment between physical exercise and education/CBT because some treatments had both characteristics. Finally, there were substantial concerns regarding the generalization of our results due to biases in the age and race of participants, types and stages of cancer, and follow-up time.
Conclusions
This systematic review and meta-analysis suggested that physical exercise, acupuncture, and alternative medicine may reduce pain in cancer survivors. In particular, the physical exercise showed a large effect on pain with moderate quality of evidence. However, 10 of the 13 included studies involved only breast cancer survivors; thus, caution should be exercised in generalizing our results. The positive results of acupuncture and alternative medicine for pain should be interpreted with caution due to the low and very low quality of evidence. The effects of treatments for numbness could not be determined due to the small number of included studies. Further research is needed, especially on the effects of widely used pharmacotherapy, including opioids, acetaminophen, nonsteroidal anti-inflammatory drugs, anticonvulsants, and antidepressants for pain and numbness in cancer survivors.
Acknowledgments
Funding: This research was supported by the National Cancer Center Research and Development Fund (Nos. 30-A-17 and 2021-A-17).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-420/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-420/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-420/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol 2003;13:248-66. [Crossref] [PubMed]
- WHO. International Agency for Research on Cancer. Globocan 2020 Population fact sheets. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Centers for Disease Control and Prevention (CDC). Cancer survivors--United States, 2007. MMWR Morb Mortal Wkly Rep 2011;60:269-72. [PubMed]
- Burton AW, Fanciullo GJ, Beasley RD, et al. Chronic pain in the cancer survivor: a new frontier. Pain Med 2007;8:189-98. [Crossref] [PubMed]
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 2016;51:1070-90.e9. [Crossref] [PubMed]
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:1941-67. [Crossref] [PubMed]
- Denlinger CS, Ligibel JA, Are M, et al. Survivorship: pain version 1.2014. J Natl Compr Canc Netw 2014;12:488-500. [Crossref] [PubMed]
- Mols F, Beijers T, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer 2014;22:2261-9. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Hamood R, Hamood H, Merhasin I, et al. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat 2018;167:157-69. [Crossref] [PubMed]
- Limakatso K, Bedwell GJ, Madden VJ, et al. The prevalence and risk factors for phantom limb pain in people with amputations: A systematic review and meta-analysis. PLoS One 2020;15:e0240431. [Crossref] [PubMed]
- Cramer JD, Johnson JT, Nilsen ML. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol Head Neck Surg 2018;159:853-8. [Crossref] [PubMed]
- Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008;44:1507-15. [Crossref] [PubMed]
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 2013;24:1443-9. [Crossref] [PubMed]
- WHO. WHO Guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Geneva: WHO, 2018.
- Yang GS, Kim HJ, Griffith KA, et al. Interventions for the Treatment of Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Nurs 2017;40:E26-41. [Crossref] [PubMed]
- Cochrane Training. Cochrane handbook for systematic reviews of interventions, version 6.3, 2022. Available online: https://training.cochrane.org/handbook/current/
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol 2018;18:25. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [Crossref] [PubMed]
- Ellison N, Loprinzi CL, Kugler J, et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J Clin Oncol 1997;15:2974-80. [Crossref] [PubMed]
- Gewandter JS, Mohile SG, Heckler CE, et al. A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer 2014;22:1807-14. [Crossref] [PubMed]
- Shapiro AC, Adlis SA, Robien K, et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat 2016;155:501-12. [Crossref] [PubMed]
- Peng N, Yu M, Yang G, et al. Effects of the Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: A randomized, controlled clinical trial. Breast 2018;37:18-27. [Crossref] [PubMed]
- De Groef A, Devoogdt N, Van Kampen M, et al. Effectiveness of Botulinum Toxin A for Persistent Upper Limb Pain After Breast Cancer Treatment: A Double-Blinded Randomized Controlled Trial. Arch Phys Med Rehabil 2018;99:1342-51. [Crossref] [PubMed]
- Kim YH, Park S, Lee M, et al. Effect of a pH-Balanced Vaginal Gel on Dyspareunia and Sexual Function in Breast Cancer Survivors Who Were Premenopausal at Diagnosis: A Randomized Controlled Trial. Obstet Gynecol 2017;129:870-6. [Crossref] [PubMed]
- Mao JJ, Liou KT, Baser RE, et al. Effectiveness of Electroacupuncture or Auricular Acupuncture vs Usual Care for Chronic Musculoskeletal Pain Among Cancer Survivors: The PEACE Randomized Clinical Trial. JAMA Oncol 2021;7:720-7. [Crossref] [PubMed]
- Crew KD, Capodice JL, Greenlee H, et al. Pilot study of acupuncture for the treatment of joint symptoms related to adjuvant aromatase inhibitor therapy in postmenopausal breast cancer patients. J Cancer Surviv 2007;1:283-91. [Crossref] [PubMed]
- Lu W, Giobbie-Hurder A, Freedman RA, et al. Acupuncture for Chemotherapy-Induced Peripheral Neuropathy in Breast Cancer Survivors: A Randomized Controlled Pilot Trial. Oncologist 2020;25:310-8. [Crossref] [PubMed]
- Burch JB, Ginsberg JP, McLain AC, et al. Symptom Management Among Cancer Survivors: Randomized Pilot Intervention Trial of Heart Rate Variability Biofeedback. Appl Psychophysiol Biofeedback 2020;45:99-108. [Crossref] [PubMed]
- Prinsloo S, Novy D, Driver L, et al. Randomized controlled trial of neurofeedback on chemotherapy-induced peripheral neuropathy: A pilot study. Cancer 2017;123:1989-97. [Crossref] [PubMed]
- Alberts NM, Leisenring WM, Flynn JS, et al. Wearable Respiratory Monitoring and Feedback for Chronic Pain in Adult Survivors of Childhood Cancer: A Feasibility Randomized Controlled Trial From the Childhood Cancer Survivor Study. JCO Clin Cancer Inform 2020;4:1014-26. [Crossref] [PubMed]
- Kelleher SA, Fisher HM, Winger JG, et al. Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support Care Cancer 2021;29:5361-9. [Crossref] [PubMed]
- Smith SK, MacDermott K, Amarasekara S, et al. Reimagine: a randomized controlled trial of an online, symptom self-management curriculum among breast cancer survivors. Support Care Cancer 2019;27:1775-81. [Crossref] [PubMed]
- Lengacher CA, Reich RR, Paterson CL, et al. Examination of Broad Symptom Improvement Resulting From Mindfulness-Based Stress Reduction in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol 2016;34:2827-34. [Crossref] [PubMed]
- Shergill Y, Rice DB, Khoo EL, et al. Mindfulness-Based Stress Reduction in Breast Cancer Survivors with Chronic Neuropathic Pain: A Randomized Controlled Trial. Pain Res Manag 2022;2022:4020550. [Crossref] [PubMed]
- Bao T, Zhi I, Baser R, et al. Yoga for Chemotherapy-Induced Peripheral Neuropathy and Fall Risk: A Randomized Controlled Trial. JNCI Cancer Spectr 2020;4:pkaa048. [Crossref] [PubMed]
- Tsai CL, Liu LC, Liao CY, et al. Yoga versus massage in the treatment of aromatase inhibitor-associated knee joint pain in breast cancer survivors: a randomized controlled trial. Sci Rep 2021;11:14843. [Crossref] [PubMed]
- Cantarero-Villanueva I, Fernández-Lao C, Fernández-de-Las-Peñas C, et al. Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial. Pain Med 2012;13:1509-19. [Crossref] [PubMed]
- Cantarero-Villanueva I, Cuesta-Vargas AI, Lozano-Lozano M, et al. Changes in Pain and Muscle Architecture in Colon Cancer Survivors After a Lumbopelvic Exercise Program: A Secondary Analysis of a Randomized Controlled Trial. Pain Med 2017;18:1366-76. [Crossref] [PubMed]
- Fields J, Richardson A, Hopkinson J, et al. Nordic Walking as an Exercise Intervention to Reduce Pain in Women With Aromatase Inhibitor-Associated Arthralgia: A Feasibility Study. J Pain Symptom Manage 2016;52:548-59. [Crossref] [PubMed]
- Fu MR, Axelrod D, Guth AA, et al. A Web- and Mobile-Based Intervention for Women Treated for Breast Cancer to Manage Chronic Pain and Symptoms Related to Lymphedema: Results of a Randomized Clinical Trial. JMIR Cancer 2022;8:e29485. [Crossref] [PubMed]
- Nyrop KA, Callahan LF, Cleveland RJ, et al. Randomized Controlled Trial of a Home-Based Walking Program to Reduce Moderate to Severe Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors. Oncologist 2017;22:1238-49. [Crossref] [PubMed]
- Baglia ML, Lin IH, Cartmel B, et al. Endocrine-related quality of life in a randomized trial of exercise on aromatase inhibitor-induced arthralgias in breast cancer survivors. Cancer 2019;125:2262-71. [Crossref] [PubMed]
- Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, et al. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain 2012;28:113-21. [Crossref] [PubMed]
- Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, et al. Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 2016;122:3166-74. [Crossref] [PubMed]
- Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 2015;33:1104-11. [Crossref] [PubMed]
- Paulo TRS, Rossi FE, Viezel J, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes 2019;17:17. [Crossref] [PubMed]
- McNeely ML, Parliament MB, Seikaly H, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer 2008;113:214-22. [Crossref] [PubMed]
- Castro-Martín E, Ortiz-Comino L, Gallart-Aragón T, et al. Myofascial Induction Effects on Neck-Shoulder Pain in Breast Cancer Survivors: Randomized, Single-Blind, Placebo-Controlled Crossover Design. Arch Phys Med Rehabil 2017;98:832-40. [Crossref] [PubMed]
- De Groef A, Van Kampen M, Vervloesem N, et al. Effect of myofascial techniques for treatment of persistent arm pain after breast cancer treatment: randomized controlled trial. Clin Rehabil 2018;32:451-61. [Crossref] [PubMed]
- Rangon FB, Koga Ferreira VT, Rezende MS, et al. Ischemic compression and kinesiotherapy on chronic myofascial pain in breast cancer survivors. J Bodyw Mov Ther 2018;22:69-75. [Crossref] [PubMed]
- Serra-Añó P, Inglés M, Bou-Catalá C, et al. Effectiveness of myofascial release after breast cancer surgery in women undergoing conservative surgery and radiotherapy: a randomized controlled trial. Support Care Cancer 2019;27:2633-41. [Crossref] [PubMed]
- Conejo I, Pajares B, Alba E, et al. Effect of neuromuscular taping on musculoskeletal disorders secondary to the use of aromatase inhibitors in breast cancer survivors: a pragmatic randomised clinical trial. BMC Complement Altern Med 2018;18:180. [Crossref] [PubMed]
- Eaton LH, Beck SL, Jensen MP. An Audio-Recorded Hypnosis Intervention for Chronic Pain Management in Cancer Survivors: A Randomized Controlled Pilot Study. Int J Clin Exp Hypn 2021;69:422-40. [Crossref] [PubMed]
- Bao T, Baser R, Chen C, et al. Health-Related Quality of Life in Cancer Survivors with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Clinical Trial. Oncologist 2021;26:e2070-8. [Crossref] [PubMed]
- Couto N, Monteiro D, Cid L, et al. Effect of different types of exercise in adult subjects with fibromyalgia: a systematic review and meta-analysis of randomised clinical trials. Sci Rep 2022;12:10391. [Crossref] [PubMed]
- Sanford NN, Sher DJ, Butler SS, et al. Prevalence of chronic pain among cancer survivors in the United States, 2010-2017. Cancer 2019;125:4310-8. [Crossref] [PubMed]
- Jairam V, Yang DX, Verma V, et al. National Patterns in Prescription Opioid Use and Misuse Among Cancer Survivors in the United States. JAMA Netw Open 2020;3:e2013605. [Crossref] [PubMed]
- Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014;32:1739-47. [Crossref] [PubMed]
- Paice JA, Portenoy R, Lacchetti C, et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:3325-45. [Crossref] [PubMed]
- Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;CD007566. [PubMed]
- Roberts KE, Rickett K, Feng S, et al. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev 2020;1:CD012988. [Crossref] [PubMed]
- Boing L, Vieira MCS, Moratelli J, et al. Effects of exercise on physical outcomes of breast cancer survivors receiving hormone therapy - A systematic review and meta-analysis. Maturitas 2020;141:71-81. [Crossref] [PubMed]
- Lu G, Zheng J, Zhang L. The effect of exercise on aromatase inhibitor-induced musculoskeletal symptoms in breast cancer survivors:a systematic review and meta-analysis. Support Care Cancer 2020;28:1587-96. [Crossref] [PubMed]
- Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev 2005;CD000335. [Crossref] [PubMed]
- O'Riordan C, Clifford A, Van De Ven P, et al. Chronic neck pain and exercise interventions: frequency, intensity, time, and type principle. Arch Phys Med Rehabil 2014;95:770-83. [Crossref] [PubMed]
- Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, et al. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomed Res Int 2017;2017:2356346. [Crossref] [PubMed]
- Izquierdo-Alventosa R, Inglés M, Cortés-Amador S, et al. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int J Environ Res Public Health 2020;17:3634. [Crossref] [PubMed]
- Valenza MC, Rodríguez-Torres J, Cabrera-Martos I, et al. Results of a Pilates exercise program in patients with chronic non-specific low back pain: a randomized controlled trial. Clin Rehabil 2017;31:753-60. [Crossref] [PubMed]
- Tekur P, Nagarathna R, Chametcha S, et al. A comprehensive yoga programs improves pain, anxiety and depression in chronic low back pain patients more than exercise: an RCT. Complement Ther Med 2012;20:107-18. [Crossref] [PubMed]
- Hayden JA, Ellis J, Ogilvie R, et al. Some types of exercise are more effective than others in people with chronic low back pain: a network meta-analysis. J Physiother 2021;67:252-62. [Crossref] [PubMed]
- Wewege MA, Booth J, Parmenter BJ. Aerobic vs. resistance exercise for chronic non-specific low back pain: A systematic review and meta-analysis. J Back Musculoskelet Rehabil 2018;31:889-99. [Crossref] [PubMed]
- Sielski R, Rief W, Glombiewski JA. Efficacy of Biofeedback in Chronic back Pain: a Meta-Analysis. Int J Behav Med 2017;24:25-41. [Crossref] [PubMed]
- Patel K, Sutherland H, Henshaw J, et al. Effects of neurofeedback in the management of chronic pain: A systematic review and meta-analysis of clinical trials. Eur J Pain 2020;24:1440-57. [Crossref] [PubMed]
- Hilton L, Hempel S, Ewing BA, et al. Mindfulness Meditation for Chronic Pain: Systematic Review and Meta-analysis. Ann Behav Med 2017;51:199-213. [Crossref] [PubMed]
- Wang L, Zhang L, Yang L, et al. Effectiveness of pain coping skills training on pain, physical function, and psychological outcomes in patients with osteoarthritis: A systemic review and meta-analysis. Clin Rehabil 2021;35:342-55. [Crossref] [PubMed]
- Mu J, Furlan AD, Lam WY, et al. Acupuncture for chronic nonspecific low back pain. Cochrane Database Syst Rev 2020;12:CD013814. [PubMed]
- Makary MM, Lee J, Lee E, et al. Phantom Acupuncture Induces Placebo Credibility and Vicarious Sensations: A Parallel fMRI Study of Low Back Pain Patients. Sci Rep 2018;8:930. [Crossref] [PubMed]
- Piras A, La Vecchia M, Boldrini L, et al. Radiofrequency thermoablation (RFA) and radiotherapy (RT) combined treatment for bone metastases: a systematic review. Eur Rev Med Pharmacol Sci 2021;25:3647-54. [PubMed]
- Rezaei Haddad A, Hayley J, Mostofi A, et al. Stereotactic Radiofrequency Thalamotomy for Cancer Pain: A Systematic Review. World Neurosurg 2021;151:225-34.e6. [Crossref] [PubMed]
- Pinheiro da Silva F, Moreira GM, Zomkowski K, et al. Manual Therapy as Treatment for Chronic Musculoskeletal Pain in Female Breast Cancer Survivors: A Systematic Review and Meta-Analysis. J Manipulative Physiol Ther 2019;42:503-13. [Crossref] [PubMed]
- Ughreja RA, Venkatesan P, Balebail Gopalakrishna D, et al. Effectiveness of myofascial release on pain, sleep, and quality of life in patients with fibromyalgia syndrome: A systematic review. Complement Ther Clin Pract 2021;45:101477. [Crossref] [PubMed]
- Chen Z, Wu J, Wang X, et al. The effects of myofascial release technique for patients with low back pain: A systematic review and meta-analysis. Complement Ther Med 2021;59:102737. [Crossref] [PubMed]
- Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013;309:1359-67. [Crossref] [PubMed]
- Salehifar E, Janbabaei G, Hendouei N, et al. Comparison of the Efficacy and Safety of Pregabalin and Duloxetine in Taxane-Induced Sensory Neuropathy: A Randomized Controlled Trial. Clin Drug Investig 2020;40:249-57. [Crossref] [PubMed]
- Jiang J, Li Y, Shen Q, et al. Effect of Pregabalin on Radiotherapy-Related Neuropathic Pain in Patients With Head and Neck Cancer: A Randomized Controlled Trial. J Clin Oncol 2019;37:135-43. [Crossref] [PubMed]
- Tanabe Y, Hashimoto K, Shimizu C, et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol 2013;18:132-8. [Crossref] [PubMed]
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699-707. [Crossref] [PubMed]




