Dopamine receptor antagonists
Abstract: Intractable nausea and/or vomiting is a serious and significant clinical dilemma that may greatly detract from quality of life. One of the first classes of antiemetic agents used as well as one of the commonest classes of antiemetic agents used is that of the dopamine receptor antagonists. Dopamine receptor antagonists are useful antiemetic agents, however, unfortunately, clinicians have on occasion resorted to switching from one dopamine receptor antagonist to another dopamine receptor antagonist when the initial dopamine receptor antagonist was ineffective rather than adding on a second antiemetic agent from a totally different class (e.g., 5-HT3 receptor antagonists, NK-1 receptor antagonists).
Key words: Nausea; vomiting; dopamine receptor antagonists; butyrophenones; phenothiazines; metoclopramide
Introduction
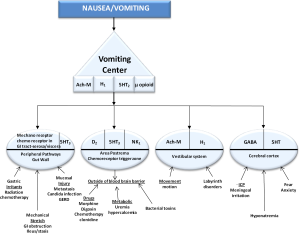
In Palliative Medicine the 4 pathways which cause the vomiting center to trigger nausea and vomiting are the chemoreceptor trigger zone (CTZ), the cortex, peripheral pathways and the vestibular system (Figure 1). The CTZ is outside the blood brain barrier and is exposed to toxins, such as chemotherapy or candida infections in the blood stream and cerebral spinal fluid which triggers vomiting. In the CTZ, D2, 5HT3 and NK1 neuroreceptors are present. Dopamine receptor antagonists (DRA) work in this neural pathway. Clozapine was the first atypical antipsychotic and DRA. Antipsychotics/DRA’s are known to be used for nausea and vomiting. They work by blocking dopamine receptors which are a class of metabotropic G protein-coupled receptors that are prominent in the vertebrate central nervous system. The neurotransmitter dopamine is the primary endogenous ligand for dopamine receptors.
Dopamine receptor antagonists are interesting antiemetic agents. DRAs are by far the most commonly used antiemetic agents, especially in palliative medicine. Although the reasons why DRAs are so popular are not entirely certain, it is likely that the two major reasons are: (I) DRAs are relatively inexpensive and (II) DRAs are the oldest antiemetic agents, and so they have the “longest track record” and also the highest patient years, as well as being the most familiar agents to clinicians and to senior teaching professors who “pass on” their preferences for DRAs to their students and junior assistant professors. DRAs may be the most popular class among the antiemetic agents; however, they probably should not be at the top of the popularity list since they may be one of the least effective (especially at the usual doses employed), as well as the antiemetic class with the most frequent adverse effects and most significant adverse effects. Furthermore, it is unfortunate that clinicians may resort to switch from one DRA that was ineffective to another DRA rather than adding a second antiemetic agent from a totally different class (e.g., 50HT3 receptor antagonists, NK-1 receptor antagonists).
Butyrophenones
Droperidol (Inapsine) and haloperidol (Haldol) are butyrophenones and two dopamine receptor antagonists that are used in palliative medicine quite frequently for treatment of nausea and vomiting. The pharmacokinetics and pharmacodynamics of haloperidol administration by different routes is an important consideration as the side effects are determined by its route of administration. Haloperidol is extensively metabolized by hepatic enzymes, partially by the CYP450 family, and there are active, inactive and toxic metabolites. Only 1% is excreted unchanged in the urine. Its metabolism is complex. Haloperidol’s metabolism consists of glucuronidation to an inactive metabolite (50-60%), reduction (and back oxidation) to reduced-haloperidol (an active metabolite) (23%) and N-dealkylation to a pyridium metabolite (a toxic metabolite) (20-30%). As a result of haloperidol’s variable metabolism, haloperidol has a variable half-life (12-35 hours). Reduced-haloperidol is not detected after intravenous administration for even longer than after oral administration which suggests that first pass metabolism is responsible for increased reduced-haloperidol concentrations.
Due to its strong antidopaminergic action, it is classified as a highly potent neuroleptic. The peripheral antidopaminergic effects at the CTZ, accounts for its strong antiemetic activity. The peripheral effects also lead to the relaxation of the gastric sphrincter muscle.
Droperidol is a short-acting butyrophenone derivative with minimal side effects when compared to haloperidol. It has minimal effects on the respiratory system and has sedative properties that last from 2 to 6 hours. It has minimal anticholinergic properties. It can be used only parenterally, unlike haloperidol which can be used orally, SC, IV or IM. Droperidol was widely believed to be more effective against nausea than vomiting, but the analysis of IMPACT data and reanalysis of a previous meta-analysis suggested that droperidol was equally effective in reducing both nausea and vomiting (1).
Although there is a black box warning for droperidol, as with haloperidol, due to prolongation of the QTc interval, prolongation is felt to be dose dependent. ECG changes not accompanied by dysrhythmias, does not seem to be a reason to avoid the use of this drug (2). Patients with preexisting conduction defects or prolongation of QTc interval may be at risk to develop dysrhythmias after droperidol administration because disposition dysrhythmias has been reported to increase after admistration of a QTc interval-prolonging drug.
Metoclopramide (REGLAN)
Metoclopramide is a dopamine antagonist with a short-life and is one of the most frequently used dopamine antagonists. However, due to the extrapyramidal symptoms that emerged during its use at high doses for the prevention of chemotherapy induced nausea and vomiting (e.g., 200 mg every 4-6 h), clinicians wound up shying away from relatively high doses (3). A 10mg dose of metoclopramide has become the most commonly used dose (4,5). A systematic review showed this conservative 10mg dose to have no clinically relevant antiemetic effect (5) and this dose is the most commonly used antiemetic dose. A dose-response study of metoclopramide by Wallenborn et al. (6) found that doses of metoclopramide higher than 10 mg (e.g., 25-50 mg) were as effective as other antiemetics.
In addition to the extrapyramidal symptoms, metoclopramide has also been associated with adverse events related to the cardiovascular system, including one patient experiencing multiple cardiac arrests after repetitive metoclopramide administrations (7).
The dosage forms of metoclopramide include: Injection, solution [preservative free]: 5 mg/mL (2 mL) [Reglan®: 5 mg/mL (2 mL, 10 mL, 30 mL)], Solution, oral: 5 mg/5 mL (0.9 mL, 10 mL, 473 mL), Tablet, oral: 5 mg, 10 mg (Reglan®: 5 mg [10 mg (scored)], Tablet, orally disintegrating, oral: [Metozolv™ ODT: 5 mg, 10 mg (mint flavor)].
Metoclopramide has a rapid absorption after oral administration (Bioavailability: oral: range: 65% to 95%) and an onset of action of 30-60 minutes after oral administration; 1-3 minutes after intravenous administration; 10-15 minutes after intravenous administration. The time to peak after administration is 102 hours and therapeutic duration of action of 1-2 hours, regardless of route. Its protein binding is about 30%. The half-life elimination in patients with normal renal function is children: ~4 hours; and adults: 5-6 hours (may be dose dependent), Roughly about 85% of metoclopramide is excreted in the urine.
Metoclopramide oral disintegrating tablets for postoperative nausea and vomiting prophylaxis: I.M., I.V. Metozolv™ ODT; Reglan® (unlabeled route): 10-20 mg near end of surgery. Note: guidelines discourage use of 10 mg metoclopramide (as well as standard clinical doses of ginger root or cannabinoids) as being ineffective for postoperative nausea and vomiting (PONV) (8); however, a comparative study indicates that higher doses (e.g., 20 mg) may be efficacious (9). Orally-disintegrating tablets may be administered on an empty stomach at least 30 minutes prior to food, and they should not be removed from packaging until time of administration. If tablet breaks or crumbles while handling, discard and remove new tablet. Using dry hands, patients should place tablet on tongue and allow to dissolve. I.M. or I.V. routes of administration are alternatives but generally suboptimal. The dosing in renal impairment with a creatinine clearance (Clcr) <40 mL/minute is to administer 50% of normal dose.
Contraindications to the administration of metoclopramide include: hypersensitivity to metoclopramide or any component of the formulation; GI obstruction, perforation or hemorrhage; pheochromocytoma; history of seizures or concomitant use of other agents likely to increase extrapyramidal reactions, depression, Extrapyramidal symptoms (EPS), Neuroleptic malignant syndrome (NMS), and Tardive dyskinesia. Metoclopramide has a U.S. Boxed Warning: may cause tardive dyskinesia, which is often irreversible; duration of treatment and total cumulative dose are associated with an increased risk.
Prochlorperazine (compazine)
Prochlorperazine is a piperazine phenothiazine antipsychotic which block postsynaptic mesolimbic dopaminergic receptors in the brain and has antiemetic effects by its antagonist actions in the D2 dopamine receptors in the chemoreceptor trigger zone. It also exhibits alpha-adrenergic blocking effect on α1 receptros and may depress the release of hypothalamic and hypophyseal hormones.
Prochlorperazine is utilized for the management of severe nausea and vomiting of various etiologies (e.g., postoperative, acute migraine, toxins, radiation, or cytotoxic drugs). Nausea and vomiting treatment with prochlorperazine may be via various routes: Oral: usually, 5 or 10 mg 3 or 4 times daily (Dosages >40 mg daily should be used only in resistant cases); Rectal: 25 mg suppository prochlorperazine twice daily, and IV: 2.5-10 mg. For control of severe nausea and vomiting during surgery: 5-10 mg IV given 15-30 minutes before induction of anesthesia. If necessary, repeat initial dose once before surgery.
Geriatric patients with dementia-related psychosis treated with antipsychotic agents are at an increased risk of death. Analyses of 17 placebo-controlled trials in geriatric patients mainly receiving atypical antipsychotic agents revealed an approximate 1.6- to 1.7-fold increase in mortality compared with that in patients receiving placebo.
The onset of prochlorperazine generally takes 30-40 minutes (conventional tablet), 60 minutes (rectal suppository), or 10-20 minutes (IM administration), and the antiemetic effects roughly have a duration of 3-4 hours (conventional tablet, rectal suppository, or IM administration). Prochlorperazine exhibits weak anticholinergic effects, moderate sedative effects, strong extrapyramidal effects, and strong antiemetic effects. It also has peripheral and/or central antagonistic activity against α-adrenergic, serotonergic, histaminergic H1, and muscarinic receptors.
To control acute symptoms during or after surgery, usually 5-10 mg IV, repeated once, if necessary; single IV doses of the drug should not exceed 10 mg. If prochlorperazine is administered intramuscularly the initial dose should be 5-10 mg; if necessary, initial dose may be repeated every 3 or 4 hours, but total dosage should not exceed 40 mg daily. For control of severe nausea and vomiting during surgery: 5-10 mg IM given 1-2 hours before induction of anesthesia. If necessary, dose may be repeated once, 30 minutes after the initial dose. To control acute symptoms during or after surgery: 5-10 mg IM, repeated once in 30 minutes, if necessary.
Contraindications to prochlorperazine administration include: Comatose states or in the presence of large amounts of CNS depressants (e.g., alcohol, barbiturates, opiates), pediatric surgery, 100dfgchildren <2 years of age or <9 kg, 100dfgchildren with conditions for which dosage has not been established, 100dfg and known hypersensitivity to prochlorperazine or other phenothiazines.
Perphenazine (TRILAFON)
Perphenazine is a relatively high potency phenothiazine that blocks dopamine 2 (D2) receptors predominantly but also may possess antagonist actions at histamine 1 (H1) and cholinergic M1 and alpha 1 adrenergic receptors in the vomiting center leading to reduced nausea and vomiting. It has less risk of sedation and orthostatic hypotension but greater risks of extrapyramidal symptoms than with low potency phenothiazines. The potency of its antiemetic effects are intermediate. It is available as an oral tablet in strengths 2, 4, 8, and 18 mg as well as in an injectable formulation - 5 mg/mL. After intramuscular injection the initial effect may be seen after 10 minutes, with peak effects occurring after 1-2 hours.
Perphenazine is well absorbed after oral administration. The time to peak after oral administration is 1-3 hours with the time to peak of the metabolite 7-hydroxyperphenzaine 2-3 hours. Perphenazine has a half-life elimination of 9-12 hours and its metabolite 7-hydroxyperphenazine of 10-19 hours.
Perphenazine is extensively hepatic to metabolites via sulfoxidation, hydroxylation, dealkylation, and glucuronidation; primarily metabolized by CYP2D6 to N-dealkylated perphenazine, perphenazine sulfoxide, and 7-hydroxyperphenazine (active metabolite with 70% of the activity of perphenazine) and excreted in the urine and feces.
Contraindications to the use of perphenazine include: hypersensitivity to perphenazine or any component of the formulation (cross-reactivity between phenothiazines may occur); severe CNS depression (comatose or patients receiving large doses of CNS depressants); subcortical brain damage (with or without hypothalamic damage); bone marrow suppression; blood dyscrasias; and liver damage. The prevalence rate of tardive dyskinesia may be 40% in elderly; development of the syndrome and the irreversible nature are proportional to duration and total cumulative dose over time. Extrapyramidal reactions are more common in elderly with up to 50% developing these reactions after 60 years of age. Drug-induced Parkinson’s syndrome occurs often; akathisia is the most common extrapyramidal reaction in elderly.
Promethazine (PHENERGAN)
Promethazine is a phenothiazine derivative which is also a potent histamine receptor H1 antagonist and an antiemetic. Its mechanisms of action include: blocking postsynaptic mesolimbic dopaminergic receptors in the brain; strong alpha-adrenergic blocking effects decreasing the release of hypothalamic and hypophyseal hormones; competing with histamine for the H1-receptor; muscarinic-blocking effects, and reducing stimuli to the brainstem reticular system.
The antiemetic dose of promethazine is 12.5-25 mg every 4-6 hours as needed. Promethazine is available as the hydrochloride in multiple formulations including: injectable solution, suppositories, tablets, and syrup (6.25 mg/5 mL). The oral route is generally the preferred route of administration, however, if the oral or rectal routes are unavailable; there may be circumstances in which the parenteral route of administration is a very reasonable therapeutic option. Intramuscular (I.M.) Administration is the preferred parenteral route of administration (although parenteral administration is not ideal). If promethazine is administered intramuscularly, it should be administered into a deep muscle.
Intravenous (I.V.) administration is not the preferred route as severe tissue damage may occur. The solution for injection should be administered in a maximum concentration of 25 mg/mL (however, more dilute solutions are recommended). If administered I.V., it should be administered via a running I.V. line at the port farthest from the patient’s vein, or through a large bore vein (not hand or wrist). Clinicians should consider administering it over 10-15 minutes (maximum: 25 mg/minute) and discontinuing immediately if burning or pain occurs with administration. Promethazine has a U.S. Boxed Warning-Promethazine injection can cause severe tissue injury (including gangrene) regardless of the route of administration. Tissue irritation and damage may result from perivascular extravasation, unintentional intra-arterial administration, and intraneuronal or perineuronal infiltration. In addition to gangrene, adverse events reported include tissue necrosis, abscesses, burning, pain, erythema, edema, paralysis, severe spasm of distal vessels, phlebitis, thrombophlebitis, venous thrombosis, sensory loss, paralysis, and palsies. Surgical intervention including fasciotomy, skin graft, and/or amputation has been necessary in some cases. The preferred route of administration is by deep I.M. injection. Subcutaneous administration is contraindicated. Discontinue intravenous injection immediately with onset of burning and/or pain and evaluate for arterial injection or perivascular extravasation. Although there is no proven successful management of unintentional intra-arterial injection or perivascular extravasation, sympathetic block and heparinization have been used in the acute management of unintentional intra-arterial injection based on results from animal studies. Furthermore, the injectable solution may contain sodium metabisulfite; which may cause allergic reactions.
There is also a Pediatric U.S. Boxed Warning-Respiratory fatalities have been reported in children <2 years of age. Use contraindicated in children <2 years. In children ≥2 years, use the lowest possible dose; other drugs with respiratory depressant effects should be avoided. Avoid use in children who may have Reyes’ syndrome or hepatic disease as adverse reactions caused by promethazine may be confused with signs of primary disease.
Promethazine has an onset of action of about 20 minutes if administered orally or intramuscularly and about 5 minutes if administered intravenously. Its duration of action is usually 4-6 hours (but can be up to 12 hours). The oral absorption of promethazine is rapid and complete. There is a large first pass effect limiting systemic oral bioavailability (10) which is about 25%. The time to maximum serum concentration is 6.7-8.6 hours after suppositories and 4.4 hours after syrup (11). Hepatic metabolism of promethazine is predominantly by hydroxylation via CYP2D6 and N-demethylation via CYP2B6 (10). The half-life elimination for I.M. administration is about 10 hours; 9-16 hours for I.V. administration; and 16-19 hours (range: 4-34 hours) for suppositories or syrup (11) with urinary excretion.
Contraindications to the use of promethazine include: allergy or hypersensitivity to promethazine, any H1 antihistamine, and/or any phenothiazine (cross-reactivity between phenothiazines may occur) or any component of the formulation; coma; treatment of lower respiratory tract symptoms (e.g., asthma); children <2 years of age; intra-arterial or subcutaneous administration. Concerns related to adverse effects include: altered cardiac conduction, anticholinergic effects, extrapyramidal symptoms, neuroleptic malignant syndrome, orthostatic hypotension, photosensitivity, sedation, impaired core body temperature regulation, lowered seizure threshold, cholestatic juandice, tardive dyskinsesia (especially in patients with Parkinson’s disease), and the potential for serious skin injury if administered parenterally.
Trimethobenzamide (Tigan)
Trimethobenzamide is a (non-phenothiazine) benzamide antiemetic that acts centrally to block D2 receptors, thereby inhibiting the medullary chemoreceptor trigger zone by blocking emetic impulses to the vomiting center.
Trimethobenzamide is available as: Capsules, oral, as hydrochloride: 300 mg [Tigan®: 300 mg], Injection, solution, as hydrochloride [Tigan®: 100 mg/mL (20 mL)], or Injection, solution, as hydrochloride (preservative free) [Tigan®: 100 mg/mL (2 mL)].
The dose for nausea/vomiting is Oral: 300 mg 3-4 times/day, or I.M.: 200 mg 3-4 times/day, and for postoperative nausea and vomiting (PONV) is I.M.: 200 mg, followed 1 hour later by a second 200 mg dose.
The oral bioavailability of trimethobenzamide is 60% to 100%. The time to peak is about 45 minutes after oral administration and; I.M. about 30 minutes after intramuscular administration. The onset action of trimethobenzamide for antiemetic effects is 10-40 minutes after oral administration and; 15-35 minutes after intramuscular administration. The duration of action is 3-4 hours. The half-life elimination is 7-9 hours. Metabolism is via oxidation with the formation of the metabolite trimethobenzamide N-oxide. Excretion is via the urine (30% to 50%, as unchanged drug within 48-72 hours).
Contraindications to trimethobenzamide are hypersensitivity to trimethobenzamide or any component of the formulation. Also, injection is contraindicated in children.
Summary
Persistent nausea and vomiting can dramatically detract from quality of life. Dopamine receptor antagonists (DRAs) are the oldest and most frequently utilized antiemetic agents. DRAs tends to be at best intermediate in antiemetic effectiveness and are the antiemetics with the most frequent and significant adverse effects. When the effectiveness of DRAs is suboptimal, some clinicians on occasion will switch to a different DRA; however, generally adding an antiemetic agent from a totally different class is a better approach.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Apfel CC, Cakmakkaya OS, Frings G, et al. Droperidol has comparable clinical efficacy against both nausea and vomiting. Br J Anaesth 2009;103:359-63.
- Lischke V, Behne M, Doelken P, et al. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg 1994;79:983-6.
- George E, Hornuss C, Apfel CC. Neurokinin-1 and novel serotonin antagonists for postoperative and postdischarge nausea and vomiting. Curr Opin Anaesthesiol 2010;23:714-21.
- Gralla RJ, Itri LM, Pisko SE, et al. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med 1981;305:905-9.
- Henzi I, Walder B, Tramèr MR. Metoclopramide in the prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized, placebo-controlled studies. Br J Anaesth 1999;83:761-71.
- Wallenborn J, Gelbrich G, Bulst D, et al. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: randomized double blind multicentre trial. BMJ 2006;333:324.
- Bentsen G, Stubhaug A. Cardiac arrest after intravenous metoclopramide: a case of five repeated injections of metoclopramide causing five episodes of cardiac arrest. Acta Anaesthesiol Scand 2002;46:908-10.
- Gan TJ, Meyer TA, Apfel CC, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2007;105:1615-28, table of contents.
- Quaynor H, Raeder JC. Incidence and Severity of Postoperative Nausea and Vomiting are Similar After Metoclopramide 20 mg and Ondansetron 8 mg Given by the End of Laparoscopic Cholecystectomies. Acta Anaesthesiol Scand 2002;46:109-13.
- Sharma A, Hamelin BA. Classic Histamine H1 Receptor Antagonists: A Critical Review of Their Metabolic and Pharmacokinetic Fate from a Bird’s Eye View. Curr Drug Metab 2003;4:105-29.
- Strenkoski-Nix LC, Ermer J, DeCleene S, et al. Pharmacokinetics of Promethazine Hydrochloride After Administration of Rectal Suppositories and Oral Syrup to Healthy Subjects. Am J Health Syst Pharm 2000;57:1499-505.