
Achievement of a durable response with eribulin for relapsed cardiac metastasis of uterine leiomyosarcoma after surgery: a case report and literature review
Introduction
Uterine leiomyosarcoma (U-LMS), arising from the myometrium, is a rare type of tumor accounting for approximately 3% of all uterine malignancies (1). It is an aggressive tumor type linked to a high risk of recurrence and metastasis to distant major organs (e.g., lungs, liver, bones), as well as poor prognosis. Although cardiac metastasis of this tumor type is extremely rare, it is often associated with life-threatening conditions (2). The most effective therapy for cardiac metastasis of U-LMS is surgical resection; however, indications for surgery are limited because of anatomical problems and the poor general condition of patients (3). Other treatment options are radiotherapy and chemotherapy. Nevertheless, due to the rarity of this malignancy, there is currently no consensus on the treatment strategy.
Herein, we present the first case of relapsed cardiac metastasis of U-LMS after surgical resection successfully treated with eribulin. The treatment resulted in decreased tumor burden and improvement of symptoms originating from the cardiac metastasis. The case is presented in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-536/rc).
Case presentation
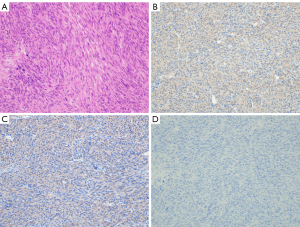
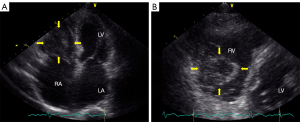
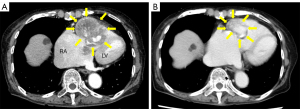
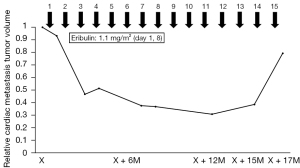
A 69-year-old female with a medical history of U-LMS was treated with surgery and radiotherapy in 1992. Ten years later, she was diagnosed with colonic metastasis and underwent surgery. The resected tumor was compatible with metastatic LMS, which was pathologically diagnosed in a tertiary referral hospital. Nineteen years after the initial treatment, she suffered from chest pain, persistent atrial fibrillation, and hypotension. In May 2011, she was diagnosed with a metastatic cardiac tumor (41 mm × 34 mm) located in the right ventricle, which blocked the right ventricular outflow tract. The patient underwent marginal surgical resection to prevent sudden death. According to the pathological examination, the cardiac tumor was a LMS; based on her medical history, the lesion was a metastatic tumor which originated from the uterus. The boundary between the tumor and surrounding tissue was unclear; as a result, the surgical margin was positive. It was impossible to confirm whether the tumor infiltrated the right atrium directly. After the operation, the symptoms originating from the cardiac tumor were improved, including atrial fibrillation. Atrial fibrillation may be induced by right atrial pressure load. Three years after cardiac operation, the patient presented with symptoms of dyspnea, tachycardia, and easy fatigability. In May 2014, she was referred to the Department of Medical Oncology at the Sapporo Medical University School of Medicine for assessment. Certified pathologists reviewed the resected cardiac tumor in our hospital. The diagnosis was U-LMS with positivity for α-smooth muscle actin (Figure 1A-1D). Therefore, the patient was diagnosed with a relapsed cardiac tumor of U-LMS. Treatment was initiated with 80% of the doxorubicin plus ifosfamide (AI) regimen (i.e., doxorubicin 30 mg/m2 on days 1 and 2; ifosfamide 2,000 mg/m2 on days 1–5) every 3 weeks to avoid the cardiotoxicity induced by doxorubicin. Following two cycles of treatment, examination through CT revealed progressive disease (PD) in the cardiac metastasis. Given the poor response to the AI regimen, which was not effective against easy fatigability caused by the cardiac metastasis, the patient was next treated with the GD regimen (i.e., gemcitabine 900 mg/m2 on days 1 and 8, docetaxel 70 mg/m2 on day 8) every 3 weeks. This regimen did not suppress tumor growth and failed to improve symptoms. The patient was subsequently treated with pazopanib (400 mg/day), which resulted in severe general malaise and intolerance to this drug. Therefore, we decided to administer eribulin as fourth-line chemotherapy. A thorough investigation was conducted prior to the administration of eribulin. Echocardiography detected a solid mass in the right ventricle, which reached the right atrium over the tricuspid valve and impaired right ventricle movement (Figure 2A,2B). An enhanced CT scan confirmed the presence of a mass (size: 61 mm × 42 mm) in the right ventricle, reaching the right atrium (Figure 3A). Further examination with chest-abdominal-pelvic CT did not reveal the presence of other metastatic tumors. In May 2016, treatment with eribulin (1.1 mg/m2) was initiated; the dose was reduced by 20% because the patient’s Eastern Cooperative Oncology Group (ECOG) performance status was 2. Eribulin was administered intravenously on days 1 and 8 every 21 days until disease progression. Follow-up CT after 11 cycles of treatment with eribulin revealed that the cardiac tumor volume was reduced by 70% compared with that measured before the administration of this agent (Figure 3B). We have revealed that increased levels of p-AKT were associated with resistance to eribulin in soft-tissue sarcoma cell lines (4). Therefore, we performed immunohistochemical analyses for p-AKT in the tumor. The tumor cells did not express p-AKT (Figure 1D). In terms of toxicity, the patient developed grade 2 anemia and grade 4 neutropenia, which were manageable with transfusion and administration of granulocyte colony-stimulating factor. Unfortunately, several pauses of treatment with eribulin were needed due to neutropenia and personal reasons. Notably, treatment with eribulin improved the symptoms caused by the cardiac metastatic tumor (i.e., dyspnea, tachycardia, and malaise), and the performance status of the patient improved from 2 to 0. As a result, her quality of life recovered to status reported prior to the relapsed cardiac metastasis. However, in October 2017, a CT scan revealed PD in the cardiac metastasis and new liver metastases after 15 cycles of treatment with eribulin (Figure 4). Because of disease progression, the patient was treated with trabectedin (0.8 mg/m2). After eight cycles of treatment, the patient was diagnosed with PD. Subsequently, she was re-treated with pazopanib (200 mg/day). Treatment with trabectedin and pazopanib did not improve the symptoms related to the cardiac tumor and quality of life. Unfortunately, the patient expired in June 2019, 8 years after the initial diagnosis of cardiac metastasis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Cardiac metastasis of soft-tissue sarcoma is associated with a dismal prognosis, as it leads to heart failure and sudden death. However, a few patients have been diagnosed with cardiac metastasis and treated. In the last decade, only 11 cases of cardiac metastasis of U-LMS have been reported in the literature (1-3,5-12) (Table 1). Generally, this condition presents as part of systemic metastases. If permitted by the general condition of the patient, surgical resection is the optimal option for the management of cardiac metastasis, which may prevent sudden death (12). Consequently, our patient initially underwent surgery (13). This case presented with extremely rare conditions; the tumor metastasized to the colon and the heart. Such tumors may have an undetermined biological feature. Thus, further investigations (e.g., RNA sequencing) may be needed to clarify their biological profile.
Table 1
| No. | Case | Age (y) | Sites of other metastasis | Treatment for cardiac metastasis | Regimen for cardiac metastasis | Survival from cardiac metastasis | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Nguyen et al., 2012 (1) | 56 | Lung | Surgery | None | 2 w | Alive |
| 2 | Marak et al., 2013 (5) | 49 | Lung | Surgery | None | Not described | Not described |
| 3 | Tunio et al., 2014 (6) | 57 | Vagina, bladder, lung, liver, subcutaneous tissue, lymph node | Radiotherapy, chemotherapy | Not described | 2 m | Alive |
| 4 | Barış et al., 2015 (7) | 56 | Abdomen | Surgery | None | Not described | Not described |
| 5 | Lv et al., 2015 (8) | 43 | None | Surgery | None | 1 y | Alive |
| 6 | Szczeklik et al., 2015 (9) | 47 | None | Surgery | None | 2 m | Alive |
| 7 | Artioli et al., 2016 (3) | 55 | Lung | Chemotherapy | 1st EPI + IFM, 2nd GEM + DOC, 3rd Trabectedin, 4th Pazopanib, cardiac metastasis, 5th GEM + DTIC | 3 m | Alive |
| 8 | Karass et al., 2016 (2) | 51 | Lung, abdomen, muscle, bone, stomach, duodenum, colon, brain | No treatment | GEM + DOC (before cardiac metastasis) | 10 d | Dead |
| 9 | Zmaimita et al., 2017 (10) | 57 | Lung | Chemotherapy | DXR | Not described | Not described |
| 10 | Sripariwuth et al., 2018 (11) | 56 | Bone | Surgery | None | 1 m | Alive |
| 11 | Maebayashi et al., 2020 (12) | 49 | Lung | Surgery, chemotherapy | 1st GEM + DOC, 2nd DXR | 7 m | Alive |
| 12 | Our case | 69 | Colon | Surgery, chemotherapy | 1st DXR + IFM, 2nd GEM + DOC, 3rd Pazopanib, 4th Eribulin, 5th Trabectedin, 6th Pazopanib | 8 y (5 y from cardiac metastasis relapse) | Dead |
y, years; m, months; w, weeks; d, days; DOC, docetaxel; DTIC, dacarbazine; DXR, doxorubicin; EPI, epirubicin; GEM, gemcitabine; IFM, ifosfamide.
Due to its rarity, treatment strategies for unresectable and/or relapsed cardiac metastasis of U-LMS are not yet established. Most of these patients receive palliative chemotherapy primarily with the GD regimen or a doxorubicin-containing regimen (2,3,10,12) (Table 1). In addition, it has been revealed that pazopanib improves PFS in patients with LMS (14). Therefore, we selected the AI regimen, GD regimen, and pazopanib as first-, second-, and third-line treatments for this patient, respectively. A phase 3 trial demonstrated that treatment with eribulin significantly improved overall survival (OS) in patients with liposarcoma who had received at least two previous systemic chemotherapies, including the administration of an anthracycline (15). As a result, in 2016, use of eribulin was approved in the United States of America and the European Union for the treatment of patients with unresectable/metastatic liposarcoma whose disease was refractory to an anthracycline-containing regimen. However, according to a subgroup analysis from a phase 3 study, eribulin exerted a similar effect to that of dacarbazine in patients with LMS in terms of OS and progression-free survival (PFS) (16). Of note, eribulin can be administered to patients with all subtypes of soft tissue sarcomas in Japan. Recently, two case reports described that treatment with eribulin as fourth-line therapy resulted in good responses in patients with refractory U-LMS, whose PFS was approximately 6–9 months (17,18). Surprisingly, in our patient, the reduction in tumor volume induced by eribulin was sustained for 17 months and improved her general condition without the development of severe adverse events (Figure 3). Possible reasons for the 5-year survival of this patient after the detection of the cardiac metastasis relapse are the successful treatment with eribulin and the sequential administration of several chemotherapeutics. Moreover, the functions of her major organs were maintained during the clinical course.
Thus far, biomarkers of sensitivity to treatment with eribulin have not been identified. However, it appears that wild-type tumor protein p53 and mutated alpha-thalassemia/mental retardation, X-linked (ATRX) were associated with shorter PFS in patients with LMS who received eribulin (19). Of note, we have previously described that elevated levels of p-AKT in several soft-tissue sarcoma cell lines were associated with the development of resistance to eribulin in vivo and in vitro (4). Additionally, we have reported that a patient with liposarcoma lacking expression of p-AKT in the tumor cells achieved a CR following treatment with eribulin (20). As expected, immunohistochemical analysis did not reveal expression of p-AKT in the tumor (Figure 1D). Hence, a favorable clinical outcome after treatment with eribulin could be expected. Further investigations are warranted to identify molecular markers which may predict response to treatment with eribulin.
Our findings suggest that eribulin is an effective and accessible treatment option for cardiac metastasis of U-LMS in patients with life-threatening conditions. Moreover, the expression levels of p-AKT in U-LMS may be a good predictive biomarker for response to treatment with eribulin. A prospective study is warranted to clarify the significance of p-AKT as a biomarker for sensitivity to eribulin.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-536/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-536/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-536/coif). KT received lecture fees from Eisai, Janssen, Chugai, Ono, Otsuka, Daiichi Sankyo and Sanofi. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nguyen SK, Wong F. Right atrial metastasis of uterine leiomyosarcoma causing obstructive shock. Curr Oncol 2012;19:e292-4. [Crossref] [PubMed]
- Karass M, Mondal P, Alkayem M, et al. A rare presentation of acute heart failure secondary to aggressive uterine leiomyosarcoma metastatic to the myocardium initially diagnosed as hypertrophic obstructive cardiomyopathy. Ann Transl Med 2016;4:374. [Crossref] [PubMed]
- Artioli G, Borgato L, Calamelli S, et al. Unusual cardiac metastasis of uterine leiomyosarcoma: case report and literature review. Tumori 2016; [Crossref] [PubMed]
- Hayasaka N, Takada K, Nakamura H, et al. Combination of eribulin plus AKT inhibitor evokes synergistic cytotoxicity in soft tissue sarcoma cells. Sci Rep 2019;9:5759. [Crossref] [PubMed]
- Marak CP, Ponea AM, Alappan N, et al. Uterine leiomyosarcoma manifesting as a tricuspid valve mass. Case Rep Oncol 2013;6:119-26. [Crossref] [PubMed]
- Tunio MA, Al-Asiri M, Fareed MM. Uterine leiomyosarcoma metastasizing to the heart. J Coll Physicians Surg Pak 2014;24:S20-1. [PubMed]
- Barış VÖ, Özcan ÖU, Gerede DM, et al. Case images: Giant left ventricular metastasis of uterine leiomyosarcoma mimicking acute coronary syndrome. Turk Kardiyol Dern Ars 2015;43:496. [PubMed]
- Lv Y, Pang X, Zhang Q, et al. Cardial leiomyosarcoma with multiple lesions involved: a case report. Int J Clin Exp Pathol 2015;8:15412-6. [PubMed]
- Szczeklik M, Gupta P, Amersey R, et al. Reconstruction of the right atrium and superior vena cava with extracellular matrix. J Card Surg 2015;30:351-4. [Crossref] [PubMed]
- Zmaimita H, Samlali H, Rachdi A, et al. Cardiac Metastasis of Uterine Leiomyosarcoma: Case Report and Literature Review. Oncol Cancer Case Rep 2017;3:123. [Crossref]
- Sripariwuth A, Xu B, Shih-Lin HS, et al. Multimodality Cardiac Imaging Assessment of a Large Metastatic Pericardial Leiomyosarcoma. CASE (Phila) 2018;2:156-62. [Crossref] [PubMed]
- Maebayashi A, Nagaishi M, Nakajima T, et al. Successful surgical treatment of cardiac metastasis from uterine leiomyosarcoma: A case report and literature review. J Obstet Gynaecol Res 2020;46:795-800. [Crossref] [PubMed]
- Magishi K, Izumi Y, Shimizu N. Metastatic Leiomyosarcoma Causing Right Ventricular Outflow Stenosis. Jpn J Cardiovasc Surg 2012;41:191-4. [Crossref]
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86. [Crossref] [PubMed]
- Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629-37. [Crossref] [PubMed]
- Blay JY, Schöffski P, Bauer S, et al. Eribulin versus dacarbazine in patients with leiomyosarcoma: subgroup analysis from a phase 3, open-label, randomised study. Br J Cancer 2019;120:1026-32. [Crossref] [PubMed]
- Fujimoto E, Takehara K, Tanaka T, et al. Uterine leiomyosarcoma well-controlled with eribulin mesylate. Int Cancer Conf J 2018;8:33-8. [Crossref] [PubMed]
- Aliberti S, Miano S, Tolomeo F, et al. Response to eribulin in a patient with metastatic uterine leiomyosarcoma: a case report. Future Oncol 2020;16:15-9. [Crossref] [PubMed]
- Wozniak A, Boeckx B, Modave E, et al. Molecular Biomarkers of Response to Eribulin in Patients with Leiomyosarcoma. Clin Cancer Res 2021;27:3106-15. [Crossref] [PubMed]
- Nakamura H, Takada K, Emori M, et al. Complete Response To Eribulin In A Patient With Unresectable Liposarcoma: A Case Report And Implications Of New Biomarkers. Intern Med 2022; Epub ahead of print. [Crossref] [PubMed]


