
Intradural disc herniation at the L2/3 level: a case report and literature review
Introduction
Intradural lumbar disc herniation (ILDH) is special type of lumbar disc herniation in which the lumbar nucleus pulposus prolapses and enters the dura mater. This type of disease was first reported by Dandy in 1942; its incidence is highest in people aged 50–60 years, and it occurs in the lumbar 4/5 segment (1). In 2001, Mut et al. divided it into types A and B. Type A is the intradural type (more common at lumbar 4/5), and type B is the intrathecal nerve root type (more common at lumbar 5/sacral 1) (2). Difficult diagnosis and high misdiagnosis rate may be related to low incidence, lack of typical imaging features in radiological evaluation, and easily mimicked as metastatic disease, arachnoid cysts, infection or intradural extramedullary tumors. Because its preoperative diagnosis is difficult, it is easy to miss the diagnosis or missed diagnosis, affecting the postoperative efficacy. An ILDH at lumbar 2/3 was recently treated at our hospital. Microscopically assisted laminectomy, dura mater incision to remove the intradural disc, pedicle screw fixation regardless of fusion, surgical results are usually favorable. It is important to remove the total intradural disc without damaging the nerve, as residual protrusion is associated with poor postoperative outcomes. ILDH occurs below the lumbar 3/4 level and corresponds to the predilection level for disc herniation, mainly related to greater lower lumbar spine stress 92% of ILDH occurred below the lumbar 3/4 level, corresponds to the predilection level for disc herniation. The incidence of lumbar 2/3 was rarely low, mainly related to lower lumbar spine stress. In our report, we present a case of ILDH at lumbar 2/3 level and discuss the clinical presentations, typical imaging features, treatments and outcomes; and related literature was reviewed and analyzed. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1017/rc).
Case presentation
General conditions
The patient was a 65-year-old man who was admitted to our hospital with pain in his waist and back, and with bilateral radiating pain of lower extremities (mainly in the front of the bilateral hips, the outer thighs, and the outer calf, and accompanied by intermittent claudication) and fatigue for more than 2 weeks. The patient had previously undergone minimally invasive radiofrequency ablation of the intervertebral disc many years ago and had been free of low back pain or lower extremity pain after surgery. Magnetic resonance imaging (MRI) of the lumbar spine revealed a space-occupying lesion in the spinal canal at the level of the lumbar 2/3 intervertebral disc, and the patient was admitted to hospital for treatment.
Physical examination
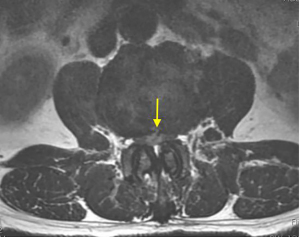
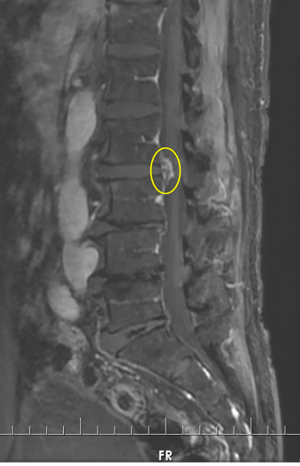
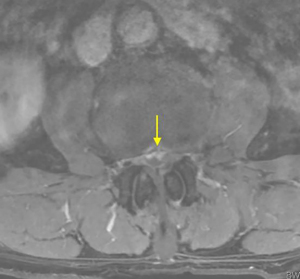
On physical examination, physiological curvature of the lumbar spine was normal, and he had positive bilateral straight leg raising test at 50° and grade 3 muscle power in great knee extensors and hip flexors, grade 4 in right ankle dorsiflexion, dorsiflexion, and ankle plantar flexor strength; and left ankle dorsiflexion, ankle plantar flexor strength were grade 3. Right knee reflex and Achilles tendon reflex disappeared, left knee reflex and Achilles tendon reflex were weakened, and the bilateral Babinski sign (–). Preoperative MRI showed a round, low-density mass in the lumbar 2/3 spinal canal on T2-weighted images (Figure 1), a loss of continuity of the posterior longitudinal ligament (PLL) in the coronal view, and mixed heterogeneous signals in the dural sac (Figure 2). Enhanced MRI showed ring enhancement sign (Figure 3) and “hawk beak” sign. of the subdural protrusion (Figure 4). The PLL was continuously lost and showed high signal, and there was a diffuse annular large central protrusion. Preoperative diagnosis of lumbar 2/3 intradural space occupying lesions: intraspinal tumor? Disc herniation? Due to progressive decrease in muscle strength in both lower limbs, posterior microscopically assisted laminectomy, dural incision of the lumbar 2/3 was performed on day 2 after admission.

Surgical method
Under general anesthesia and in the prone position, midline laminectomy was done at the lumbar 2/3 level with careful preservation of the facet joint capsule. We made a 5-cm longitudinally midline incision of dura mater and arachnoid membrane, adjust the height of the head and feet. We suspend the dura mater with “0” silk thread, then a large ILDH/fragment was encountered measuring 2.0 cm × 0.5 cm × 0.5 cm under the microscope. After carefully teasing away the fragment, we observed a swollen dural perforation (about 0.4 mm) connecting with the PLL, and the ventral side is not sutured. The adhesions between PLL and the ventral dura was extremely resistant. Dural tear repair was performed with a prolene 5-0 suture. Appropriate closure was confirmed on the table by the Valsalva manoeuvre to check for leakage of cerebrospinal fluid, and a negative pressure rubber tube was inserted for drainage.
Postoperative treatment
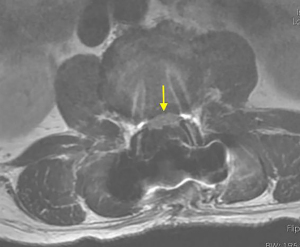
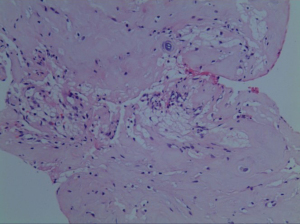
Postoperatively, the patient was kept on bed rest for 48 h, then take off the tube without cerebrospinal fluid leakage. On the first postoperative day, the radiating pain in both lower extremities was significantly relieved. Re-examination of lumbar spine MRI showed that the intradural nucleus pulposus was completely removed, the cauda equina nerve signal was uniform (Figure 5), and an oblong low-density air signal was seen behind the lumbar 4 vertebral body. On the third postoperative day, the patient walked normally with the waist circumference on, the feeling of fatigue basically disappeared, the urine and urination were normal, and he recovered and was discharged from the hospital smoothly. The pathological findings were degenerated fibrous connective tissue and cartilage tissue (Figure 6), consistent with ILDH. He returned to normal life and work 3 months after surgery, and 1-year follow-ups showed that walking gait was normal, muscle strength of both lower extremities was grade 5, physiological reflexes of both lower extremities were normal, and the bowel and bladder were normal.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Epidemiology
ILDH is relatively rare, and many scholars, such as Aydin et al., have reported that the proportion of ILDH in lumbar intervertebral disc herniation is 0.04–0.33% (3). In 2013, Ducati et al. reported that its proportion was 1.76% (4). When the dural sac was adhered and fixed, or the number of intervertebral disc fragments was fewer than expected, ILDH was considered and the dura was incised for exploration, so the incidence rate was higher than that reported in other studies. Luo et al. proposed that since Dandy first reported ILDH in 1942 until 2018, about 160 cases of ILDH have been reported successively around the world. The incidence of lumbar disc herniation in patients aged 50–60 years was reported to be 2.2%, and the incidence in patients aged 40–50 years was 0.2%, of which males account for about 72% (5). Oztürk et al. found that 92% of ILDH occurred in the lumbar spine (6). The incidence of lumbar 4/5 segments was about 55%, followed by lumbar 3/4 in 16%, and lumbar 5/sacral 1 in 10%. The incidence of lumbar 2/3 and lumbar 1/2 was low, thoracic spine accounts for 5%, the most frequent segments were T6–11, and cervical spine accounted for only 3% (6). ILDH is often mistaken for cauda equina syndrome. Ducati et al. proposed that the incidence of cauda equina syndrome in general lumbar disc herniation is about 0.5–1%, while the cauda equina syndrome in ILDH accounts for 30–60%, of which 0.5–1% occurs in the early stage of the herniation (4).
Pathogenesis
The pathogenesis of ILDH still remains unclear and it is currently thought that it may be:
- The herniated disc extrudes the PLL and the ventral dura mater, causing exudation from obstructed extra-mural venous return and increased tension in the dura mater after compression, resulting in aseptic inflammatory edema. Floeth an Herdmann. found obvious venous enlargement and thickening of the arachnoid around the ventral dural defect through preoperative computed tomography (CT) and MRI, suggesting that chronic degenerative disc disease and chronic erosive aseptic inflammatory reaction between the PLL and the dura mater are the cause of ILHD (7). At our hospital, preoperative contrast-enhanced MRI of the lumbar 2/3 segment ILDH showed a high-intensity inflammatory reaction in the ventral dura. During the operation, hyperplasia and swelling of the veins near the ventral dural cleft were also seen.
- The anterior wall of the ventral dura and the PLL are closely fixed due to congenital factors or various acquired pathological factors. Yildizhan et al. studied the autopsies of 20 adults without low back pain and neonates and found that the multi-segment ventral dura and the PLL often had dense and firm adhesions, with the most common adhesions occurring in the lumbar 4/5 segment, proving that the human body has some congenital adhesions, suggesting that it is related to the pathogenesis of intradural ILDH that is prevalent in the lumbar 4/5 segment (8). Spencer et al. confirmed the presence of dural ligaments in a cadaveric study of adults, which anchor the dura and nerve roots to the periosteum of the vertebral body at the exit of their nerve roots, resulting in tight fixation of the nerve roots and a predisposition to intrathecal herniation of the nerve roots type ILDH, which occurs in the lumbar 5/sacral 1 segment (9). In their review of 40 autopsies, Blikra found that part of the ventral dura mater was densely adhered to the PLL, which could be caused by traumatic stimulation, history of intervertebral disc surgery, history of epidural anesthesia, ossification of the PLL, or intervertebral disc herniation (10). D’Andrea et al. proposed through a retrospective analysis of 9 patients with ILDH that tight junctions between the annulus fibrosus, PLL, and dura mater may be due to congenital spinal stenosis, narrowing of the epidural space, congenital or iatrogenic thinning of the dura mater, and previous history of chronic inflammation or surgery may lead to adhesion fixation (11). In our case, he had a previous history of minimally invasive radiofrequency disc ablation, during which significant adhesion and fixation of the ventral dura mater were seen.
- Repeated mechanical action and chemical corrosion of the herniated intervertebral disc tissue and adhesion tissue, and then by the shear force of the intervertebral disc, the PLL ruptures, the adhesively fixed ventral dura is ruptured, and the free nucleus pulposus enters the dura lower cavity. Sharma et al. reported the rupture of the PLL and the ventral dura after acute external force with a chronic history of ILDH in different segments, resulting in the broken nucleus pulposus enter the dural sac (12). Our patient with lumbar 2/3 segment ILDH had a tear of the PLL on preoperative MRI, and a dural rupture was also detected during the operation. However, Kim et al. reported a special type of ILDH at lumbar 2/3 in 2018, during which adhesion of the ventral dura mater to the PLL was observed, but no significant rupture of the dura mater, PLL, and annulus fibrosus was observed, and the specific mechanism may need to be further studied (13). Sarliève et al. first reported a case of ILDH in the lumbar 4/5 segment with a history of lumbar spine surgery in 2004, and proposed that the breakdown products of β-protein and histamine in the fragmented nucleus pulposus had a chemical corrosion effect on the dural sac, and the surrounding fibrosis and cicatricial arachnoiditis cause the intervertebral disc to protrude into the dural sac after rupture of the dural sac (14).
Imaging
MRI is the gold standard for diagnosing ILDH, and contrast-enhanced MRI is the gold standard.
Aprígio et al. proposed that the typical MRI signs of ILDH include the following:
- Moderate or low-signal intensity on both T1- and T2-weighted imaging (15).
- Rim enhancement sign on enhanced MRI due to chronic inflammation. There is hyperplasia of granulation tissue and the formation of peripheral new microvessels, ring enhancement after contrast medium, and no enhancement of the central nucleus pulposus. However, intraspinal tumors often show heterogeneous enhancement in the central area due to rich blood supply on enhanced MRI, and the signal is inconsistent with the surrounding intervertebral disc tissue (5-7,14-17).
- Due to the loss of continuity of the PLL, the fibrocartilage forms a beak-like, hawk-beak protrusion, called “Hawk beak” sign (16). Crivelli and Dunet showed clearly the ruptured PLL, the disc protruding into the dura mater by three-dimensional (3D) high resolution constructive interference in steady state (CISS), and this sign also helped to diagnose ILDH (18).
- The nucleus pulposus of the intervertebral disc ruptures the ventral dura but does not break through the arachnoid, forming a “Y” of the dura and arachnoid. Sasaji et al. described the MRI showing subdural extra-arachnoid disc herniation showing “Y” sign (19). In our case, the lumbar 2/3 ILDH-enhanced MRI showed a prominent rim enhancement sign around the nucleus pulposus and an “Hawk beak” sign after the rupture of the PLL. Combined with the intraoperative findings, it was considered that the prominent nucleus pulposus had broken through the arachnoid, so there was no typical “Y” sign.
CT examination is not highly sensitive to ILDH, but Hidalgo-Ovejero et al. found that 46% of ILDH patients can have gas in the spinal canal on CT scan, and the incidence was six times higher in those with intraspinal gas than in those without intraspinal gas, suggesting that the gas in the spinal canal can help the diagnosis of ILDH (20). However, Mercier et al. proposed that if the density of intradural lesions is the same as that of the intervertebral disc [64 Hounsfield units (HU)], based on CT, and there is no enhancement after contrast agent, ILDH should be considered (21). Myelography is helpful for the diagnosis of ILDH, which is usually manifested by complete occlusion of the contrast medium in the lesion segment. Schisano et al. suggested that this typical sign of ILDH exists but cannot be completely characterized because it is also possible for other intraspinal mass lesions (22).
Cerebrospinal fluid examination is also helpful for the diagnosis of ILDH. Smith et al. reported that the presence of macrophages that phagocytose fibrocartilage components in the cerebrospinal fluid is helpful for the diagnosis of ILDH (23). Intraoperative ultrasonography has important clinical value for the diagnosis of ILDH. Sharma et al. proposed that intraoperative ultrasound not only helps to determine the nature of the intradural mass but also shows the exact location and number of the intervertebral disc in the intradural space (12).
During surgery, when the expected fragment of the intervertebral disc nucleus pulposus cannot be found in the epidural, the dura is clearly not damaged but the cerebrospinal fluid flows spontaneously, and the X-ray fluoroscopy confirms that the surgical segment is correct, the possibility of intradural disc herniation should be considered at this time (4,5).
Treatment
In 2020, Morozumi et al. reported a case of ILDH without surgical treatment, which was absorbed spontaneously 13 months after the onset of symptoms, which is a real rare case (24). Sakai et al. reported a case of lumbar 2/3 intradural disc herniation with recurrence 9 months after surgery, and spontaneous resorption was confirmed by MRI 9 months later (25). However, Borota et al. reported a patient with similar recurrence at lumbar 4/5 who still had significant symptoms after 8 months and opted for surgical removal under the microscope, and the postoperative recovery was good (26).
Most doctors prefer early surgery for ILDH, including Koç et al., who reported that early surgery is necessary because neurological prognosis is closely related to the duration of preoperative symptoms (27). Montalvo Afonso et al. reported that, due to the high incidence of ILDH complicated by cauda equina syndrome, early surgical treatment of ILDH was necessary (28).
The standard procedure is hemilaminectomy or total laminectomy followed by microscopically assisted incision of the dural sac to separate the nerve roots and remove the disc fragments. Low et al. proposed that, in the treatment of upper lumbar ILDH, due to anatomical reasons, such as spinal stenosis, short lamina, nearly vertical facet joints, and a full dural sac, it is recommended to perform microscopic surgery after total laminectomy (29). Ashraf and Babar reported a case of lumbar 4/5 giant intervertebral disc herniation with cauda equina syndrome (30). Routine laminectomy and durotomy were performed to remove free fragments, and the dura mater was closed in 1 stage. Their patient recovered without complications. Sharma had difficulty in 6 ILDH cases who underwent traditional microscopic tunnel excision, then conversion to laminectomy and successfully completed with the microscopically assisted surgery (12). Thohar Arifin et al. suggested a microsurgical approach with a median or paramedian dural incision to better observe the intradural disc and avoid damage to the nerve roots (31). Huliyappa et al. found that after the removal of the intervertebral disc in the spinal canal, further resection of the intervertebral disc was not required, and intervertebral fusion might not be required (32). In our case, we perform the standard laminectomy followed by microscopically assisted incision of the dural sac to separate the nerve roots and remove the disc fragments. The patient smoothly returned to normal life and work 3months without complications.
With the development of spinal endoscopy, some scholars have used spinal endoscopy to treat intradural disc herniation. In 2018, Kim et al. reported the first case of lumbar 2/3 ILDH via transforaminal endoscopic approach, and a good clinical effect was obtained after an 8-month follow-up (13). In 2017, Huliyappa et al. reported a case of lumbar 5/sacral 1 segment ILDH treated with posterior percutaneous spinal endoscopy (32). In 2020, Moon et al. reported a case in which the lumbar 2/3 intervertebral disc was removed under a laparoscope (33). No free intervertebral disc was observed after facetoplasty. The free intervertebral disc in the capsule was observed when the adhesive capsule was separated. The operation was completed by changing to the microscope after endoscopic removal was difficult (33).
Poor prognostic factors
- Symptoms persist too long lead to poor prognosis. Kataoka et al. found that the degree of neurological recovery was closely related to the preoperative course of disease, and early surgical treatment generally has a better prognosis (34). In 2014, Kobayashi et al. reported a case of thoracic 12/lumbar 1 ILDH with a duration of 1 month. Surgical treatment was unsuccessful, and was this was believed to be related to the long course of the disease and the fragility of the conus medullaris (35).
- Combined cauda equina syndrome, or residual symptoms of partial cauda equina nerve damage, can lead to a poor prognosis. Choi et al. reported that 62% of their ILDH cases with cauda equina syndrome recovered slowly, 38% recovered poorly, and the recovery time was at least 3 weeks and up to 32 months (16). Arrigo et al. reported that, when combined with cauda equina syndrome, surgery within 48 hours of the onset of symptoms is recommended for good prognosis (36).
- Residual protrusion and history of previous lumbar surgery suggests poor prognosis. D’Andrea et al. reported 9 cases of ILDH with 12-year follow-up. About 67% of their cases were able to return to work normally, and 33% had residual neurological dysfunction, urgency of urination, sensory loss, and muscular dystrophy. It is proposed that there is a correlation between prognosis and residual protrusion and history of lumbar surgery (11).
In summary, ILDH is a rare intervertebral disc herniation in clinical practice, and it is difficult to differentiate it from intraspinal tumors. Typical rim enhancement sign, “Hawk beak” sign and “Y” sign are important features of MRI in diagnosing ILDH, however, intraspinal tumors often show heterogeneous enhancement in the central area due to rich blood supply. Intraspinal gas is also helpful in CT diagnosis of ILDH. Prompt microscopically assisted laminectomy, dura mater incision to remove the intradural disc, pedicle screw fixation regardless of fusion, surgical results are usually favorable. Spinal endoscopic techniques have also been gradually applied to the removal of herniated intradural discs.
Acknowledgments
Funding: This article was funded by the Guangdong Province Basic and Applied Basic Research Fund (No. 2021A1515220157).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1017/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1017/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dandy WE. Recent advances in the diagnosis and treatment of ruptured intervertebral disks. Ann Surg 1942;115:514-20. [Crossref] [PubMed]
- Mut M, Berker M, Palaoğlu S. Intraradicular disc herniations in the lumbar spine and a new classification of intradural disc herniations. Spinal Cord 2001;39:545-8. [Crossref] [PubMed]
- Aydin MV, Ozel S, Sen O, et al. Intradural disc mimicking: a spinal tumor lesion. Spinal Cord 2004;42:52-4. [Crossref] [PubMed]
- Ducati LG, Silva MV, Brandão MM, et al. Intradural lumbar disc herniation: report of five cases with literature review. Eur Spine J 2013;22:S404-8. [Crossref] [PubMed]
- Luo D, Ji C, Xu H, et al. Intradural disc herniation at L4/5 level causing Cauda equina syndrome: A case report. Medicine (Baltimore) 2020;99:e19025. [Crossref] [PubMed]
- Oztürk A, Avci E, Yazgan P, et al. Intradural herniation of intervertebral disc at the level of Lumbar 1-Lumbar 2. Turk Neurosurg 2007;17:134-7. [PubMed]
- Floeth F, Herdmann J. Chronic dura erosion and intradural lumbar disc herniation: CT and MR imaging and intraoperative photographs of a transdural sequestrectomy. Eur Spine J 2012;21:S453-7. [Crossref] [PubMed]
- Yildizhan A, Paşaoğlu A, Okten T, et al. Intradural disc herniations pathogenesis, clinical picture, diagnosis and treatment. Acta Neurochir (Wien) 1991;110:160-5. [Crossref] [PubMed]
- Spencer RR, Jahnke RW, Hardy TL. Dissection of gas into an intraspinal synovial cyst from contiguous vacuum facet. J Comput Assist Tomogr 1983;7:886-8. [Crossref] [PubMed]
- Blikra G. Intradural herniated lumbar disc. J Neurosurg 1969;31:676-9. [Crossref] [PubMed]
- D'Andrea G, Trillò G, Roperto R, et al. Intradural lumbar disc herniations: the role of MRI in preoperative diagnosis and review of the literature. Neurosurg Rev 2004;27:75-80; discussion 81-2. [Crossref] [PubMed]
- Sharma A, Singh V, Sangondimath G, et al. Intradural Disc a Diagnostic Dilemma: Case Series and Review of Literature. Asian J Neurosurg 2018;13:1033-6. [Crossref] [PubMed]
- Kim HS, Pradhan RL, Adsul N, et al. Transforaminal Endoscopic Excision of Intradural Lumbar Disk Herniation and Dural Repair. World Neurosurg 2018;119:163-7. [Crossref] [PubMed]
- Sarliève P, Delabrousse E, Clair C, et al. Intradural disc herniation with cranial migration of an excluded fragment. Clin Imaging 2004;28:170-2. [Crossref] [PubMed]
- Aprígio RM, Caramanti RL, Santos FOR, et al. Intradural disc herniation at the L1-L2 level: A case report and literature review. Surg Neurol Int 2019;10:196. [Crossref] [PubMed]
- Choi JY, Lee WS, Sung KH. Intradural lumbar disc herniation--is it predictable preoperatively? A report of two cases. Spine J 2007;7:111-7. [Crossref] [PubMed]
- Pholprajug P, Wiratapesuporn T, Satayasoontorn K, et al. Intradural disc herniation of L2/3: A case report and literature review. N Am Spine Soc J 2022;11:100138. [Crossref] [PubMed]
- Crivelli L, Dunet V. Intradural lumbar disc herniation detected by 3D CISS MRI. BMJ Case Rep 2017;2017:bcr-2017-221728. [Crossref] [PubMed]
- Sasaji T, Horaguchi K, Yamada N, et al. The specific sagittal magnetic resonance imaging of intradural extra-arachnoid lumbar disc herniation. Case Rep Med 2012;2012:383451. [Crossref] [PubMed]
- Hidalgo-Ovejero AM, García-Mata S, Gozzi-Vallejo S, et al. Intradural disc herniation and epidural gas: something more than a casual association? Spine (Phila Pa 1976) 2004;29:E463-7. [Crossref] [PubMed]
- Mercier P, Hayek G, Ben Ali H, et al. Intradural lumbar disk hernias. Apropos of 6 cases and review of the literature. Neurochirurgie 1997;43:142-7. [PubMed]
- Schisano G, Franco A, Nina P. Intraradicular and intradural lumbar disc herniation: experiences with nine cases. Surg Neurol 1995;44:536-43. [Crossref] [PubMed]
- Smith RV. Intradural disc rupture. Report of two cases. J Neurosurg 1981;55:117-20. [Crossref] [PubMed]
- Morozumi N, Aizawa T, Sasaki M, et al. Spontaneous Resorption of Intradural Lumbar Disc Herniation: A Rare Case Report. Spine Surg Relat Res 2019;4:277-9. [Crossref] [PubMed]
- Sakai T, Tsuji T, Asazuma T, et al. Spontaneous resorption in recurrent intradural lumbar disc herniation. Case report. J Neurosurg Spine 2007;6:574-8. [Crossref] [PubMed]
- Borota L, Jonasson P, Agolli A. Spontaneous resorption of intradural lumbar disc fragments. Spine J 2008;8:397-403. [Crossref] [PubMed]
- Koç RK, Akdemir H, Oktem IS, et al. Intradural lumbar disc herniation: report of two cases. Neurosurg Rev 2001;24:44-7. [Crossref] [PubMed]
- Montalvo Afonso A, Mateo Sierra O, Gil de Sagredo Del Corral OL, et al. Misdiagnosis of posterior sequestered lumbar disc herniation: report of three cases and review of the literature. Spinal Cord Ser Cases 2018;4:61. [Crossref] [PubMed]
- Low JCM, Rowland D, Kareem H. L1/2 Intradural Disc Herniation with Compression of the Proximal Cauda Equina Nerves: A Surgical Challenge. World Neurosurg 2020;142:147-51. [Crossref] [PubMed]
- Ashraf A, Babar ZU. Intradural Disc Herniation: A Case Report and Literature Review. Cureus 2020;12:e7600. [Crossref] [PubMed]
- Thohar Arifin M, Ikbar K N, Brilliantika SP, et al. Challenges in intradural disc herniation diagnosis and surgery: A case report. Ann Med Surg (Lond) 2020;58:156-9. [Crossref] [PubMed]
- Huliyappa HA, Singh RK, Singh SK, et al. Transdural herniated lumbar disc disease with muscle patch for closure of durotomy - A Brief review of literature. Neurol Neurochir Pol 2017;51:149-55. [Crossref] [PubMed]
- Moon SJ, Han MS, Lee GJ, et al. Unexpected Intradural Lumbar Disk Herniation Found During Transforaminal Endoscopic Surgery. World Neurosurg 2020;134:540-3. [Crossref] [PubMed]
- Kataoka O, Nishibayashi Y, Sho T. Intradural lumbar disc herniation. Report of three cases with a review of the literature. Spine (Phila Pa 1976) 1989;14:529-33. [Crossref] [PubMed]
- Kobayashi K, Imagama S, Matsubara Y, et al. Intradural disc herniation: radiographic findings and surgical results with a literature review. Clin Neurol Neurosurg 2014;125:47-51. [Crossref] [PubMed]
- Arrigo RT, Kalanithi P, Boakye M. Is cauda equina syndrome being treated within the recommended time frame? Neurosurgery 2011;68:1520-6; discussion 1526. [Crossref] [PubMed]
(English Language Editor: R. Scott)


