
Effects of electroacupuncture with dexmedetomidine on myocardial ischemia/reperfusion injury in rats
Introduction
Myocardial ischemia/reperfusion (I/R) injury is a complex pathophysiologic process that occurs during hypoxia/reoxygenation treatment. Free oxygen radicals are widely recognized as the mechanism underlying the injury. Myocardial I/R injury can activate the free radical chain reaction to produce more free oxygen radicals, such as reactive oxygen species (ROS), superoxide dismutase (SOD), and malondialdehyde (MDA). The free oxygen radicals further react with cellular components to cause cell structure damage and energy metabolism disturbances (1). Studies in traditional Chinese medicine (TCM) have shown that needling the Neiguan acupoint [pericardium 6 (PC6)] with electroacupuncture (EA) can increase the mitochondrial SOD and adenosine triphosphate (ATP) content of cardiomyocytes in rats with a myocardial I/R injury. Such pretreatment protects the mitochondrial structure and function, improves cardiac function and cardiomyocyte activity, and decreases the infarct area. Needling the relevant acupoints by EA has an excellent protective effect on cardiomyocytes (2-5). Sinomenine and sodium aescinate can protect the myocardium of I/R rats by inhibiting inflammation (6,7). According to studies in Western medicine, dexmedetomidine (Dex) pretreatment reduces the myocardial I/R injury area in rats and inhibits myocardial apoptosis due to the I/R injury by inhibiting endoplasmic reticulum stress, the inflammatory response, and mitochondrial oxidative stress (8-11). Both EA and Dex have been shown to be protective for cardiomyocytes; however, whether the myocardial protective effect can be enhanced by EA + Dex has rarely been reported. An in-depth investigation is needed to determine the mechanism of action. Herein we discuss the protective effects of EA + Dex against myocardial I/R injury in a rat model of myocardial I/R injury and tentatively propose the underlying mechanism. We present the following article in accordance with the ARRIVE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-969/rc).
Methods
General materials
Healthy adult male Sprague-Dawley (SD) rats, each weighing approximately 200 g, were purchased from Beijing Huafukang Biotechnology Co., Ltd. [laboratory animal license No. SCXK (Beijing) 2019-000; Beijing, China]. Animal experiments and raising animals were carried out in Yishengyuan Gene Science and Technology. Animal experiments was approved by institutional ethics board of Yishengyuan Gene Science and Technology (No. YSY-DWLL-2021085), in compliance with Chinese guidelines for the care and use of animals. A protocol was prepared before the study without registration. The reagents and instruments used for the study were as follows: inhaled sevoflurane, manufactured by Shanghai Hengrui Pharmaceutical Co., Ltd. (batch No. 20042731; Shanghai, China); small animal anesthesia machine (ZS-MV-IV series; Beijing Zhongshidichuang Science and Technology Development Co., Ltd., Beijing, China); Hwato SDZ-II Electronic Acupuncture Stimulator Machine (Suzhou, China); centrifuge machine (Eppendorf-5430; Hamburg, Germany); and a microplate reader (DNM-9602; Perlong Medical, Beijing, China).
Study methods
Building the rat model of myocardial I/R injury
The rats were weighed and anesthetized using the small animal anesthesia machine under 3% sevoflurane. After the corneal reflex had disappeared, the rats were immobilized on the operating table in a supine position. The skin of the chest was prepared, followed by conventional disinfection and spreading of the sterile hole towel. The skin was incised from the fourth intercostal space on the left, and blunt dissection of muscles was performed to open the chest cavity. The pericardium was cut open, and the heart was delivered. A thread was passed through the left auricle and the pulmonary conus for ligation 1–2 mm from the starting point of the left anterior descending artery and its branches. A pediatric tympanostomy (PE) tube was placed where the thread was passed. Finally, the thread was tied in a loose knot to complete the modeling. The myocardial I/R injury was induced by subjecting the heart to ischemia for 30 minutes, followed by reperfusion for 60 minutes. Ischemia induction was considered successful if there was a significant ST-segment elevation on electrocardiogram (ECG) and a darkened color appeared in the myocardium below the ligation line. The heart was reperfused for 60 minutes after 30 minutes of ligation. The reperfusion was considered successful upon observing local inflammatory edema, exudation, and congestion with ST-segment depression by >1/2 (3,12). The rats were sacrificed for tissue harvest 1 hour after the reperfusion procedure.
Animal grouping and treatments
A total of 50 male SD rats were randomly divided into 5 groups, with 10 rats in each group. In the sham operation group, after exposure, the heart was only threaded without ligating the left coronary artery. In the I/R group, the left coronary artery was ligated for 30 minutes, followed by reperfusion for 60 minutes to build the I/R injury model. In the EA group, the PC6 acupoint was needled by EA bilaterally 7 days before the modeling for the I/R group. The acupoint was located according to the Rat Experimental Atlas (13). The EA procedure was implemented under the following parameters: needling depth, approximately 5 mm; electric current,1 mA; and frequency, 2 Hz (3). Each electrical simulation continued until a mild limb tremor was observed. The EA was delivered once daily for 30 minutes per treatment for 7 consecutive days. The I/R injury was induced as planned 7 days later. An equal volume of normal saline was injected intraperitoneally 15 minutes before inducing the I/R injury. In the Dex group, Dex (5 µg/kg) was injected intraperitoneally 15 minutes before inducing the I/R injury (7). In the EA + Dex group, PC6 was stimulated bilaterally by EA for 7 consecutive days. Dex (5 µg/kg) was injected intraperitoneally 15 minutes before inducing the I/R injury, followed by the same modeling procedure as in the I/R group. The animals were kept in the specific-pathogen-free standard animal room, and the feeding conditions were as follows: the temperature of the animal room was 20–25 ℃, the relative humidity was 50%±10%, and the water was freely accessible.
Detection indicators
Infarct area calculation
The modeling was established over 1 hour after all specified procedures were completed. The whole heart was fixed in formaldehyde and subjected to 2,3,5-triphenyletetrazolium chloride (TTC) staining. The normal tissues stained blue, while the infarct tissues were white and not stained. The infarct area-to-total myocardial ratio was calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Determination of serum SOD, MDA, ATP, and ROS content
We obtained and centrifuged 100 g of myocardial tissues. The centrifuge tube was placed in a boiling water bath for 10 minutes, then removed, shaken well, and centrifuged at 1,301 g for 10 minutes. The supernatant was collected for detection of SOD, MDA, ATP, and ROS. All of the above indicators were determined according to the instructions provided with the kits. The kits were manufactured by the Nanjing Jiancheng Biological Engineering Research Institute (Nanjing, China).
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (IBM Corp., Chicago, IL, USA). Measurement data are expressed as the mean ± standard deviation (). Tests for normality and homogeneity of variance were performed. The means of the groups were compared by one-way analysis of variance (ANOVA). Dunnet’s test was used for multiple comparison tests. A P value <0.05 was considered statistically significant.
Results
Results of myocardial hematoxylin and eosin (HE) staining
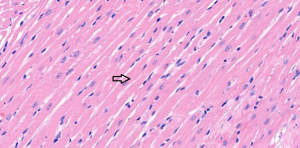
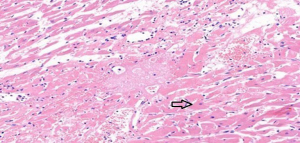
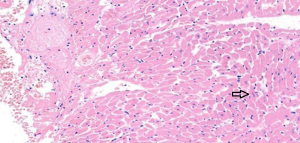
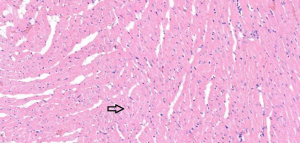
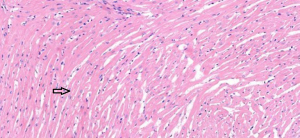
In the sham operation group, the cardiomyocytes were neatly aligned, with intact morphology, abundant cytoplasm, plump nuclei, and clear nucleoli. No abnormalities were found in the myocardial interstitium (Figure 1). In the I/R group, fractured, damaged, and swollen myocardial fibers, nuclear chromatin concentration, and unclear nucleoli were observed. Massive inflammatory cell infiltration was noted locally (Figure 2). In the EA and Dex groups, myocardial fiber fracture and swelling were less significant than the I/R group. Mild inflammatory cell infiltration was observed locally (Figures 3,4). In the EA + Dex group, the myocardial fibers were neatly aligned, and some were mildly swollen. A few myocardial fibers were fractured (Figure 5). The arrows in each figure show the observation site of pathological changes in myocardial tissue in each group.
Compared with the sham operation group, the infarct area of the I/R group was significantly increased (P<0.01). The infarct area of the Dex, EA, and EA + Dex groups were significantly decreased compared with the I/R group (P<0.01). In addition, the infarct area of the EA + Dex group was considerably smaller than the EA and Dex groups (P<0.05). There was no significant difference in the infarct area between the Dex and EA groups (P>0.05; Table 1).
Table 1
| Group | Case | Infarct area: total tissue area ratio |
|---|---|---|
| Sham group | 10 | 0 |
| I/R group | 10 | 2.32±0.04* |
| Dex group | 10 | 1.52±0.02** |
| EA group | 10 | 1.55±0.05** |
| EA + Dex group | 10 | 0.18±0.02**∆ |
Data are present as . *P<0.01 for the comparison between the I/R and sham groups; **P<0.01 for the comparison of the EA, Dex, and EA + Dex groups against the I/R group; ∆P<0.05 for the comparison of the EA + Dex and EA groups against the Dex group; P>0.05 for the comparison between the EA and Dex groups. I/R, ischemia/reperfusion; Dex, dexmedetomidine; EA, electroacupuncture; EA + Dex, electroacupuncture combined with dexmedetomidine; , mean ± standard deviation.
Changes in the serum SOD, MDA, ATP, and ROS content
Compared with the sham group, the SOD and ATP content of the I/R group decreased significantly (P<0.01), while the MDA and ROS content increased considerably (P<0.01). Compared with the I/R group, the SOD and ATP content of the Dex, EA, and EA + Dex groups increased significantly (P<0.01), while the content of the MDA and ROS decreased dramatically (P<0.01). The EA + Dex group outperformed the Dex and EA groups in all indicators (P<0.05). There were no significant differences between the Dex and EA groups in the above indicators (P>0.05; Table 2).
Table 2
| Group | Case | SOD activity (U/mL) | MDA content (mmol/L) | ATP [μmol/(g·prot)] | ROS |
|---|---|---|---|---|---|
| Sham group | 10 | 4.37±0.12 | 3.99±0.13 | 78.93±50.18 | 70.20±9.84 |
| I/R group | 10 | 2.52±0.19* | 5.73±0.03* | 14.60±13.09* | 103.26±10.83* |
| Dex group | 10 | 3.49±0.05** | 4.69±0.04** | 27.35±1.78** | 94.66±1.12** |
| EA group | 10 | 3.55±0.01** | 4.87±0.01** | 26.39±1.67** | 94.57±1.90** |
| EA + Dex group | 10 | 3.87±0.04**∆ | 4.39±0.01**∆ | 31.21±2.17**∆ | 97.20±0.47**∆ |
Data are present as . *P<0.01 for the comparison between the I/R and sham groups; **P<0.01 for the comparison of the EA, Dex, and EA + Dex groups against the I/R group; ∆P<0.05 for the comparison of the EA and Dex groups against the EA + Dex group; P>0.05 for the comparison between the EA and Dex groups. SOD, superoxide dismutase; MDA, malondialdehyde; ATP, adenosine triphosphate; ROS, rective oxygen species; I/R, ischemia/reperfusion; Dex, dexmedetomidine; EA, electroacupuncture; EA + Dex, electroacupuncture combined with dexmedetomidine; , mean ± standard deviation.
Discussion
Myocardial I/R injury is a complex pathophysiologic process that occurs during hypoxia/reoxygenation treatment. Free oxygen radicals are considered a primary cause of I/R injury (1). Under normal situations, the intracellular antioxidants can clean up the free oxygen radicals to achieve a balance between the production and removal of free oxygen radicals (1). Myocardial I/R results in the production of large amounts of ROS via the xanthine/xanthine oxidase system, activated neutrophils, and damage to the mitochondrial respiratory chain (1,2). As the balance between the production and removal of free oxygen radicals is disrupted, the cardiomyocytes are damaged structurally and functionally (1). The resulting disturbance of myocardial energy metabolism further inhibits the activity of SOD, an oxygen-free radical scavenging enzyme (1,2). Moreover, intracellular calcium overload inhibits oxidative phosphorylation, leading to an ATP synthase disturbance (1,2). The lipid peroxidation reaction increases cell membrane permeability, promoting the reaction between the free oxygen radicals and the unsaturated fatty acids to produce MDA, which further aggravates cell damage (1,2,12). Studies have shown that needling PC6 by EA increases the mitochondrial SOD and ATP content of rat cardiomyocytes with an I/R injury (2). Cardiac function and cardiomyocyte activity is significantly improved following I/R injury, reducing the infarct area and the degree of mitochondrial swelling, thus protecting mitochondrial integrity (2,3,14). Our study showed that stimulating PC6 by EA significantly increased the SOD and ATP content, while reducing MDA and ROS production. The myocardial damage caused by free oxygen radicals was alleviated, which was consistent with the findings of the aforementioned studies.
Dex is a highly selective α2-adrenergic receptor agonist and has been widely used in the perioperative setting due to its anti-sympathetic and analgesic effects, and the resulting reduction in anesthetic dosage. A growing number of studies have confirmed the vital role of Dex in I/R injury (15). The protective effect of Dex has been evidenced against cerebral I/R injury, alleviating the inflammatory and stress responses (16). Pretreatment with Dex can reduce the area of myocardial I/R injury in rats. Dex can also inhibit apoptosis of rat cardiomyocytes with I/R injury by upregulating Bcl-2 expression and downregulating Bax expression (17). Intestinal I/R injury activates the autophagic apoptosis of cells. Dex inhibits the autophagic apoptosis of cells induced by intestinal I/R injury via the HMGB1/TLR4/NF-κB pathway, thereby exerting a protective effect against intestinal I/R injury (18). We also found that Dex increased SOD and ATP content, reducing MDA and ROS production and having a protective effect against myocardial I/R injury. The above finding agrees with the viewpoint that Dex has a protective effect against I/R injury in cells.
Both EA and Dex have a protective effect against myocardial I/R injury. However, few reports have involved the EA + Dex pretreatment in I/R injury. There is also a scarcity of investigations from the perspective of the potential impact on the free oxygen radical-scavenging ability. Here, we delivered EA + Dex pretreatment in a rat model of myocardial I/R injury. The results showed that the combination treatment group had increased SOD and ATP content, while reducing MDA and ROS production to alleviate myocardial I/R injury. In addition, combination treatment outperformed either monotherapy group in all indicators.
To conclude, EA + Dex reduced MDA and ROS production and alleviated myocardial I/R injury by increasing the SOD and ATP content. Combining TCM and Western medicine may offer a new pathway to prevent and treat I/R injury.
Acknowledgments
Funding: This work was supported by the Young and Middle-Aged Scientific Research Cultivation Fund Project of Tianjin Medical Association Anesthesiology Branch (No. TJMZJJ-2018-09).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-969/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-969/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-969/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments was approved by institutional ethics board of Yishengyuan Gene Science and Technology (No. YSY-DWLL-2021085), in compliance with Chinese guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo Q, Chen SY, Zhang ZM, et al. Study on association between ischemia-reperfusion and oxygen free radicals. Shaanxi Medical Journal 2006;35:932-3, 941.
- Chang J, Sun ZR. Changes of Organizational Structure of Myocardium and SOD, MDA content in Model Rats with Ischemic Reperfusion Injury and Hyperlipemia by Acupuncture Preconditioning. Journal of Clinical Acupuncture and Moxibustion 2012;28:56-8.
- Chen S, Han YL, Wu S, et al. Electroacupuncture Preconditioning at "Neiguan"Prevents Myocardial Ischemia-reperfusion Injury in Rats by Activating p38-MAPK Pathway. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2017;46:526-30.
- Ma JN, Wu S, Li D, et al. Effect and Mechanism of Different Preconditioning Time of Electro-Needling PC6 on Cardiac Function and Insulin Resistance in Rats with MIRI. Journal of Clinical Acupuncture and Moxibustion 2021;37:83-8.
- Zhang XL, Xiang LL, Xue YX, et al. Effects of electroacupuncture pretreatment on mitochondria-mediated apoptosis in rats with myocardial ischemia reperfusion injury. Chinese Journal of Rehabilitation Medicine 2021;36:1198-205.
- Zhang F, Zheng G, Qi J, et al. Protective effect of sinomenine preconditioning on acute myocardial ischemia-reperfusion injury in rats. Chinese Journal of Immunology 2022;38:1833.
- Wen JJ, Su Z, Luo SX. Sodium aescinate reduces myocardial infarction area and no-reflow area of rats through the p38MAPK pathway. Chinese Journal of Comparative Medicine 2021;31:8-15.
- Hu XY, Huang LZ. Protective effect of dexmedetomidine pretreatment on the cardiac muscle cell in myocardial ischemia reperfusion of rat. The Chinese Journal of Clinical Pharmacology 2017;972:2032-4.
- Li C, Zhu KS, Shen JM, et al. Role of mitochondrial ATP-sensitive potassium channels in dexmedetomidine-induced attenuation of myocardial ischemia-reperfusion injury in rats. Chinese Journal of Anesthesiology 2017;37:1318-21.
- Wu ZL, Zhu Y. Dexmedetomidine Attenuates Oxidative Stress in Myocardial Ischemia-reperfusion Injury through Trx1/AMPK Pathways. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2020;49:404-7.
- Yin CZ, Chen H, Chen YJ, et al. Role of C-Jun N-terminal kinases singnaling pathway in dexmedetomidine preconditioning in reducing myocardial ischemia-reperfusion injury in isolated rat hearts. Chongqing Medicine 2021;50:3786-92.
- Deng YJ, Fu YH, Tan N, et al. Establishment and evaluation of rat heart ischemia-reperfusion injury model in vivo. Chinese Journal of Pathophysiology 2011;27:412-6.
- Hua XB, Zhou HN. Development of acupoint map of rats. Laboratory Animals and Animal Experiments 1991;1-5.
- Mu XY, Song YH, Zhang D, et al. Preconditioning of Needling PC6 Improving MIRI: A Study of Research Progress. Journal of Clinical Acupuncture and Moxibustion 2018;34:75-9.
- Behmenburg F, Pickert E, Mathes A, et al. The Cardioprotective Effect of Dexmedetomidine in Rats Is Dose-Dependent and Mediated by BKCa Channels. J Cardiovasc Pharmacol 2017;69:228-35. [Crossref] [PubMed]
- Ma HB, Yang YC, Liu JJ, et al. Effect of Dexmedetomidine on glycocalyx in mice with cerebral ischemia-reperfusion injury. China Medical Herald 2020;17:8-12, 26.
- Zhang JJ, Ji FH, Meng XW, et al. Effects of dexmedetomidine preconditioning on myocardial expression of high mobility group box-1 protein during myocardial ischemia/reperfusion injury in rats. International Journal of Anesthesiology and Resuscitation 2016;37:1063-7.
- Lu Y, He QX, Zhu YW, et al. Dexmedetomidine inhibits autophagic apoptosis induced by intestinal ischemia-reperfusion through HMGB1/TLR4/NF-κB. Modern Medical Journal 2021;49:123-8.
(English Language Editor: J. Jones)


