
A patient with advanced lung squamous cell carcinoma who failed to benefit from albumin bound paclitaxel plus pembrolizumab achieved partial response with second-line treatment of docetaxel plus pembrolizumab: a case report
Introduction
Lung cancer is the leading cause of cancer-related deaths in China and worldwide (1). Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, with squamous cell lung cancer (SqCLC) being the second common pathological type of NSCLC, accounting for about 25–30% of NSCLC cases (2). More than 60% of SqCLC patients present at an advanced stage at the time of diagnosis (3). Combined chemotherapy and immunotherapy is currently the primary first-line treatment for advanced NSCLC patients without driver gene mutations (4). Chemotherapy monotherapy (including paclitaxel, docetaxel, gemcitabine, etc.) or immunotherapy (such as pembrolizumab) are the second-line recommendations, respectively (5). However, less than 20% of patients with NSCLC benefit from second-line therapy (6). For advanced NSCLC patients with disease progression after first-line treatment, selecting the appropriate second-line regimen to maximize survival benefit has become an urgent issue. At present, for patients who have progressed on first-line platinum-containing double-drug combination immunotherapy, whether immunotherapy is applied across the line is still inconclusive. Herein, we present a case of a 53-year-old man diagnosed with stage IVA right lung squamous cell lung cancer with left lung metastasis (cT2aN1M1b). After four cycles of first-line treatment with albumin-binding paclitaxel + carboplatin combined with pembrolizumab regimen, the patient presented with disease progression. He was subsequently administered four cycles of docetaxel + carboplatin combined with pembrolizumab. The lung lesions became significantly smaller with good efficacy [reaching partial response (PR)] and the patient only experienced mild adverse reactions. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-960/rc).
Case presentation
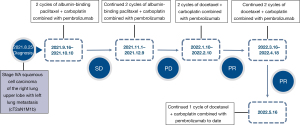
A 53-year-old man was diagnosed with a lung mass without cough, expectoration, nor hemoptysis, during his August 2021 health checkup. No family history or smoking history. Positron emission tomography/computed tomography (PET/CT) scans (performed on 2021-08-16) showed a massive soft tissue density shadow in the anterior segment of the upper lobe of the right lung, with a maximum cross-sectional diameter of 3.66 cm × 2.67 cm. A nodular high-density shadow was visible in the upper lobe of the left lung, with dimensions of 1.06 cm × 0.81 cm. High metabolic foci were found in both upper lobes. Considering the malignant lesions tended to be metastatic lesions in the left lung. There were multiple tiny nodules in both lungs without increased metabolism, and metastasis could not be excluded. Right hilar lymph node metastasis was detected. CT puncture biopsy of the upper tip bronchus of the right lung (performed on 2021-8-25) showed a medium-low differentiated squamous cell carcinoma. Genetic screening (performed on 2021-8-26) showed a TP53 EXON6 C.641A>G P (H214R) change with mutation abundance of 59.86% and microsatellite stable (MSS). Immunohistochemistry (performed on 2021-9-2) showed poorly differentiated squamous cell carcinoma (in the upper tip bronchus of the right lung) with positive staining for P40(+), Ki-67(+60%), P63(+), programmed death ligand 1 (PD-L1; SP263) [tumor proportion score (TPS) <3%], and programmed death 1 (PD-1; lymphocytes +1%). There was no previous history of underlying medical conditions. History of alcohol abuse, smoking, and familial neoplasia were denied. The diagnosis at admission was stage IVA squamous cell carcinoma of the upper lobe of the right lung, with left lung metastasis (cT2aN1M1b). The patient received first-line albumin-binding paclitaxel + carboplatin combined with pembrolizumab regimen for four cycles from September 16, 2021. The regimen consisted of albumin-binding paclitaxel (260 mg/m2) + carboplatin (AUC =6) d1 intravenous drip combined with pembrolizumab (200mg) d1 intravenous drip, q21d. The last cycle of chemotherapy was administered on December 9, 2021. Two-cycle chest enhancement CT showed stable disease (SD) and four-cycle chest enhancement CT showed anterior enlargement of the mediastinal soft tissue shadow in the upper lobe of the right lung. The efficacy evaluation was progressive disease (PD) (the mass in the anterior segment of the right lung upper lobe was 5.5 cm × 2.9 cm, larger than the anterior one) and progression free survival (PFS) was 4.0 months.
The patient received second-line docetaxel + carboplatin combined with pembrolizumab regimen for four cycles from January 10, 2022. The regimen was as follows: docetaxel (75 mg/m2) + carboplatin (AUC =6) d1 intravenous drip combined with pembrolizumab (200mg) d1 intravenous drip, q21d. The last treatment was administered on April 18, 2022. After two cycles, the evaluation was PR (43.6% reduction of the chest lesion, 3.1 cm × 1.1 cm) (Figures 1,2). During this period, myelosuppression accompanied with fever occurred after chemotherapy, and preventive leukocyte elevation therapy was given, with no other adverse events.
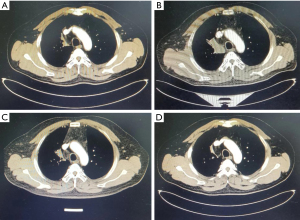
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
At present, chemotherapy, targeted therapy, and immunotherapy are the three major treatment options for patients with advanced NSCLC, and these play an important role in the first-line setting. As the second most common pathological type of NSCLC, squamous cell lung cancer has unique molecular biological characteristics (including central tumor location, elderly patients, diagnosis with advanced disease, and more complications), with low epidermal growth factor receptor (EGFR) gene mutation rates and low anaplastic lymphoma kinase (ALK) gene fusiom rates, about 2.7% and 1.5–2.5%, respectively (7-9). Therefore, only a few patients can benefit from targeted therapy. For second-line treatment, there are far fewer options. While PD-1/PD-L1 inhibitor or docetaxel/gemcitabine chemotherapy is available, the actual efficacy is unsatisfactory. Clinically, more efficient regimens with lower toxicity are urgently needed to improve patients’ survival and prognosis.
Albumin-binding paclitaxel (hereinafter referred to as “albumin paclitaxel”) and docetaxel are commonly used as first- and second-line chemotherapy drugs for advanced SqCLC, both of which belong to the taxane class of chemotherapy drugs. Taxanes can also include paclitaxel injection and paclitaxel liposome. Although the mechanisms are similar, a previous study has shown that they differ in their pharmaceutical properties and antitumor activities (10). Considering drug components and mechanisms, albumin paclitaxel is a novel paclitaxel antitumor agent, and the main active component is paclitaxel (11). It uses human albumin as a carrier and does not need polyoxyethylene castor oil as a cosolvent carrier. Tumor cells have a high metabolic uptake of albumin, which enters through the gp60 transcytosis pathway and the secreted protein acidic and rich in cysteine (SPARC) pathway. The cytotoxic drug is then released, allowing for a targeted effect. By using albumin, the allergic reaction associated with using castor oil as the paclitaxel cosolvent is avoided (12). Docetaxel is a second-generation high-efficiency taxane antitumor drug synthesized by modification of the basic structure of paclitaxel. The main active substance is a derivative of docetaxel, which blocks the cell cycle in stage M by inhibiting the normal recombination of intracellular microtubule network, thereby inhibiting the mitosis and cell proliferation of tumor cells to achieve its antitumor effect (13). Compared with traditional paclitaxel, docetaxel has a stronger affinity with tubulin at the same dose, and the combination of the two does not change the number of filaments. On the other hand, docetaxel has a longer retention time in cells, and a study has shown that docetaxel has a higher antitumor activity, which is about 2 times that of paclitaxel (14). In addition, it has been reported that docetaxel can also promote tumor cell apoptosis by down-regulating the expression of the BCL-2 gene, thereby improving the survival rate of patients. In addition, a study in vivo and in vitro has found that docetaxel has a higher affinity for the beta subunit of tubulin compared with paclitaxel, so that a higher intracellular drug concentration can be achieved, resulting in a lower frequency of drug efflux pumps, and greater tumor growth inhibition (13).
In the present case, our patient developed lung lesions after receiving four cycles of the first-line albumin-binding paclitaxel + carboplatin combined with pembrolizumab regimen. The patient was then given four cycles of the second-line docetaxel + carboplatin combined with pembrolizumab regimen and the efficacy evaluation was PR. The above-mentioned characteristics of docetaxel may explain the lack of cross-resistance to docetaxel after previous paclitaxel treatment. Unfortunately, there is a paucity of clinical studies evaluating the role of docetaxel as a second-line treatment for NSCLC patients, which limits our understanding of cross-resistance in the clinical setting (15-17). A prospective phase II study by Macedo-Pérez et al., analyzing the response to docetaxel in advanced NSCLC patients after first-line paclitaxel treatment, found that the long-term PFS of first-line paclitaxel plus platinum was associated with the improvement of PFS after second-line docetaxel treatment (18). These findings suggested that previous treatment with paclitaxel does not exclude a favorable response to docetaxel. Therefore, some patients previously treated with paclitaxel may achieve a good response to the second-line taxane treatment, possibly due to the intrinsic sensitivity of the tumor to this series of drugs.
A report by Goto et al. (19) found that previous paclitaxel use had no effect on the response or survival of patients with subsequent docetaxel treatment. Rather, the response of advanced NSCLC patients to previous chemotherapy has predictive value for subsequent docetaxel treatment. Two phase III clinical trials (20,21) also indicated that the response to second-line docetaxel was not associated with prior paclitaxel treatment exposure or efficacy. However, none of these studies evaluated clinical pathology features nor molecular differences between patients who received first-line paclitaxel and those who received other taxanes (such as docetaxel) as the second-line treatment.
With the continuous development of therapeutic methods for the treatment of NSCLC, taxanes have often been combined with other drugs in clinical practice, and drug-drug interactions may affect the therapeutic effect to a certain extent. Recent data (22-26) emphasized that chemotherapy can enhance the immunogenicity and destroy the immune resistance of the tumor and its microenvironment. In addition, chemotherapy can also induce the generation of subclone new antigen, which helps to increase tumor mutation, thus activating cellular immune responses and enhancing the sensitivity of immune checkpoint inhibitors (ICIs). Therefore, when the chemotherapy drugs kill the tumor cells, they also enable the patients to produce lymphocytes with improved function and enhance the anti-tumor immune response capability of the body (27,28). The potential synergistic antitumor effects of checkpoint inhibitors combined with chemotherapy represented by PD-1/PD-L1 have been demonstrated in multiple solid tumors (29-32). Similarly, in the PROLUNG phase II study (33), pembrolizumab plus docetaxel was shown to significantly prolong the PFS in patients with NSCLC compared to docetaxel alone. A retrospective study by Huang et al. (34) demonstrated that ICIs combined with chemotherapy or antiangiogenic agents can provide survival benefits for NSCLC patients who failed to respond to first-line therapy. A prospective phase II study in China (35) indicated that the combination of sintilimab and docetaxel significantly improved PFS and tumor response in previously treated patients with advanced NSCLC. These data support the feasibility and potential benefits of docetaxel combined with ICIs in the second-line treatment of advanced NSCLC. In the present case, on the basis of disease progression after four cycles of first-line treatment with albumin paclitaxel and pembrolizumab, the chemotherapy drug was replaced with docetaxel as second-line treatment and the disease showed a partial response trend. Whether docetaxel may exert a superior synergistic anti-tumor efficacy compared to albumin paclitaxel warrants further investigation.
Notably, genetic testing in our patient revealed the presence of a TP53 mutation with a high mutation abundance of 59.86%. Potential survival benefits have been observed with ICI therapy in patients with NSCLC harboring the KRAS/TP53 mutation or co-mutations, and thus, the TP53 mutation may be considered a potential predictive biomarker of ICI therapy outcomes (34,36). Indeed, it may provide potential evidence for showing the efficacy of PR in our patient after 8 cycles of treatment with pembrolizumab. ICIs combined with chemotherapy may be a novel treatment strategy for patients with TP53 mutations and future studies are warranted.
Second-line ICIs combined with chemotherapy may have potential clinical benefits for NSCLC patients who have failed first-line treatment. As shown in this case study, the combination of pembrolizumab and docetaxel was beneficial in a patient who had previously failed to respond to albumin paclitaxel plus pembrolizumab. This suggested that patients with advanced lung squamous cell carcinoma who fail first-line immunotherapy with paclitaxel may achieve a good response to docetaxel combined with immunotherapy, and this may be related to the differences between the pharmacological properties of albumin paclitaxel and docetaxel and their antitumor activities. Alternatively, it may be related to the different cross-resistance ranges between the two drugs. In addition, due to the presence of ICIs, the drug-drug interaction between chemotherapy and immunotherapy may also affect the therapeutic outcome. Further studies should be conducted to determine whether docetaxel may exert a superior synergistic antitumor effect compared to albumin paclitaxel. In addition, the TP53 mutation may be a promising biomarker for predicting the therapeutic effect of clinical ICIs. This may be useful in identifying the beneficiary population for chemotherapy, especially docetaxel combined with immunotherapy. However, due to the limited sample size, insufficient data, and the possible combination of various other factors, a larger patient population and specialized pharmacological and clinical studies are required in the future to confirm the potential benefits, mechanisms, and beneficiary population identified herein.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-960/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-960/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Adizie JB, Khakwani A, Beckett P, et al. Stage III Non-small Cell Lung Cancer Management in England. Clin Oncol (R Coll Radiol) 2019;31:688-96. [Crossref] [PubMed]
- Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. [Crossref] [PubMed]
- Socinski MA, Obasaju C, Gandara D, et al. Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer. J Thorac Oncol 2018;13:165-83. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Caliò A, Nottegar A, Gilioli E, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol 2014;9:729-32. [Crossref] [PubMed]
- Forbes SA, Bhamra G, Bamford S, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet 2008;Chapter 10:Unit 10.11.
- Wang J, Shen Q, Shi Q, et al. Detection of ALK protein expression in lung squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer Res 2014;33:109. [Crossref] [PubMed]
- Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release 2013;170:365-72. [Crossref] [PubMed]
- Alves RC, Fernandes RP, Eloy JO, et al. Characteristics, Properties and Analytical Methods of Paclitaxel: A Review. Crit Rev Anal Chem 2018;48:110-8. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Gligorov J, Lotz JP. Preclinical pharmacology of the taxanes: implications of the differences. Oncologist 2004;9:3-8. [Crossref] [PubMed]
- Andriguetti NB, Raymundo S, Antunes MV, et al. Pharmacogenetic and Pharmacokinetic Dose Individualization of the Taxane Chemotherapeutic Drugs Paclitaxel and Docetaxel. Curr Med Chem 2017;24:3559-82. [Crossref] [PubMed]
- Fossella FV, Lee JS, Shin DM, et al. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol 1995;13:645-51. [Crossref] [PubMed]
- Gandara DR, Vokes E, Green M, et al. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial. J Clin Oncol 2000;18:131-5. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Macedo-Pérez EO, Morales-Oyarvide V, Mendoza-García VO, et al. Long progression-free survival with first-line paclitaxel plus platinum is associated with improved response and progression-free survival with second-line docetaxel in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 2014;74:681-90. [Crossref] [PubMed]
- Goto Y, Sekine I, Yamada K, et al. Influence of previous chemotherapy on the efficacy of subsequent docetaxel therapy in advanced non-small cell lung cancer patients. J Thorac Oncol 2008;3:412-6. [Crossref] [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Bruno PM, Liu Y, Park GY, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 2017;23:461-71. [Crossref] [PubMed]
- Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725-41. [Crossref] [PubMed]
- Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. [Crossref] [PubMed]
- Wanderley CW, Colón DF, Luiz JPM, et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res 2018;78:5891-900. [Crossref] [PubMed]
- Welters MJ, van der Sluis TC, van Meir H, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016;8:334ra52. [Crossref] [PubMed]
- Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97-111. [Crossref] [PubMed]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915-28. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Arrieta O, Barrón F, Ramírez-Tirado LA, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:856-64. [Crossref] [PubMed]
- Huang D, Cui P, Huang Z, et al. Anti-PD-1/L1 plus anti-angiogenesis therapy as second-line or later treatment in advanced lung adenocarcinoma. J Cancer Res Clin Oncol 2021;147:881-91. [Crossref] [PubMed]
- Han X, Guo J, Tang X, et al. Efficacy and safety of sintilimab plus docetaxel in patients with previously treated advanced non-small cell lung cancer: a prospective, single-arm, phase II study in China. J Cancer Res Clin Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


