
Frequency of discharge of hospitalized patients with stroke to free-standing hospice facilities—a register study from Germany
Introduction
Stroke is a common disease, with an estimated 101 million prevalent cases and 12.2 million incident cases worldwide (1). It mainly affects elderly people. Ischemic stroke (IS) is the most common variant at more than 80% of cases, followed by cases of hemorrhagic origin based on intracerebral bleeding (ICB) or subarachnoid bleeding (SAB) (2). Although considerable progress has been made over recent decades in the treatment, diagnosis, and secondary prevention of stroke, severe or fatal courses of the disease are not uncommon. Approximately 11% of all deaths worldwide can be attributed to stroke (3). The mortality rates differ, sometimes significantly, depending on the nature of the stroke. For example, observational studies have put the 1-month mortality rate with IS at between 5.7% and 14.9% (4-9), with ICB at between 29.6% and 46.5% (5,10-12) and SAB at between 19.1% and 33.0% (5,13,14). Patients with cerebral hemorrhage thus have a higher mortality in comparison with IS patients. In Western industrialized countries, the majority of stroke patients die in hospital (15,16). Stroke is also the most common cause of acquired disability in adulthood. Up to 40% of survivors suffer from long-term restrictions in daily life (17-19).
In Germany, just over 300,000 cases diagnosed with stroke are hospitalized annually (20). Analyses of nationwide stroke registries show a very high rate of acute in-patient care for these patients (21-23). However, registry data from Germany also show that about 5% of all stroke patients die in hospital during acute in-patient treatment (21).
Numerous studies have emphasized the need for palliative care and the importance of including palliative care expertise in the treatment of stroke patients with severe disease courses (24-37). Respect for the patient's dignity represents an essential aspect of palliative care here. This palliative care need can manifest itself in different situations during the disease—for example, when there is an initially unfavorable status with disturbed consciousness, when there is a progressive course within the first few weeks (“progressive stroke”), or when there is a lack of response to therapeutic and/or rehabilitative measures that have been initiated. The palliative care approach in hospitals includes the provision of consultation on care for patients by a palliative care team or the transfer of patients to a palliative care unit. Depending on the patient’s condition, discharge home or to a long-term care facility with the involvement of a specialized outpatient palliative care team (SOPC) or transfer of patients to an in-patient hospice may also be considered.
Although transferring patients from the hospital to their own homes for personal, family reasons appears quite reasonable, it is not always possible to implement this model of care in reality, as the amount of care required and the intensity of the patient’s care often prove to be too burdensome for family caregivers, despite the involvement of professional outpatient support services. In such cases, patients are preferably transferred to in-patient facilities, where the quality of palliative care can vary widely. In Germany, palliative care in nursing homes is generally rated lower in comparison with in-patient hospices. The main reasons for this are the high workload and the shortage of nursing staff in nursing homes. In contrast, the staffing ratio in in-patient hospices is usually higher, and an SOPC team is also regularly involved in patient care, which is not always the case in nursing homes. Transfer to a hospice can only occur if the patient’s life expectancy is less than 6 months, and this has to be confirmed by a physician.
Few data are available worldwide on the prevalence of hospice transfer of hospitalized stroke patients (38-40). In a retrospective single-center study in the United States, Chauhan et al. analyzed data from 2,446 patients with IS (38), 4.1% of the affected patients were transferred to hospices. duPreez et al. reported data for elderly Medicare-insured patients (≥65 years) in the United States who had been hospitalized for ischemic cerebral infarction (39). Among the patients who died within the first 30 days after the event, a total of 23% had been transferred to hospices.
To date, there have been no studies in Germany on the issue of the proportion of stroke patients who are discharged to hospices after hospital admission and a severe disease course. The aim of the present study was therefore to determine the frequency of hospital discharges of stroke patients to in-patient hospices and to identify potential determinants of discharge to hospices. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-418/rc).
Methods
Study design
The present study is based on a large stroke quality assurance project, the Northwest-German Stroke Registry (Qualitätssicherung Schlaganfall Nordwestdeutschland, QSNWD), including patients from 2017 to 2020.
Northwest-German Stroke Registry
The QSNWD is one of a total of 10 stroke registries in Germany. With a total of 195 participating hospitals (as of December, 2020) from 8 states, it is the largest stroke registry in Germany. The hospitals are mainly located in the western and northern part of Germany, but hospitals from the former East German states are also included. Participation in the registry is voluntary for hospitals, but mandatory for certification as a stroke unit. Descriptive data from participating hospitals indicate a high quality of medical care for stroke patients in the acute phase of the condition (41). At the time of the study, more than two-thirds of the participating hospitals (n=145) had a certified stroke unit; systemic thrombolysis was performed in 16.9% of cases and surgical thrombectomy in 8.5%.
Observation period
The period of observation was from January 1, 2017 to December 31, 2020. The years 2017, 2018, 2019, and 2020 were intentionally combined into a single overall observation period in order to increase the rate of expected events with the target variable “hospital discharge to a hospice”. This was done due to the assumption that only few transfers per year would be documented in the stroke registry.
Patients in the stroke registry
Stroke patients aged 18 or over were included in the registry. Patients with transient ischemic attacks (TIAs) (ICD-10: G45), SAB (ICD-10: I60), ICB (ICD-10: I61), IS (ICD-10: I63), and strokes not designated as bleeding or infarction (“other”, ICD-10: I64) were documented. Patients with benign or malignant neoplasia in the brain or meninges (ICD-10: D32, D33, C70, C71, C72), patients with other cerebrovascular diseases (ICD-10: I67), and patients with traumatic head or skull injury (ICD-10: S06, S07, S08, S09) were excluded.
It was possible that patients might have been documented more than once in the stroke registry since the patient data were anonymised. It should therefore be noted that the results of this analysis apply exclusively at the case level.
The primary aim of the registry was to record acute in-patient stroke care, corresponding to the interval between the occurrence of the event and hospital admission within ≤7 days. If this definition was met, the admission situation, severity of illness, diagnosis and therapy, and reason for discharge of in-patients with stroke were documented in detail. However, the questionnaire could also be terminated prematurely by the physician if the following conditions were present: if the stroke had occurred more than 7 days earlier, or if there were other reasons (e.g., in-patient admission for early rehabilitation rather than acute treatment of a stroke, or existence of a purely palliative approach even before hospital admission). In cases of early closure of the data set, a “minimal data set (MDS)” was created that included only the items admission date, ICD-10 principal diagnosis, year of birth, sex, and reason for discharge.
Study patients
Study patients were defined as patients (≥18 years) with a principal diagnosis of IS (ICD-10: I63), ICB (ICD-10: I61), or SAB (ICD-10: I60). The following variables from the stroke registry were used for analysis: sex, age, time interval event-to-admission (≤7, >7 days), comorbidity (arterial hypertension, diabetes mellitus, atrial fibrillation, previous stroke), prehospital care (independent at home, requiring home care, institutional care), state of consciousness at admission (clear vs. reduced (somnolent, soporic or comatose)), National Institutes of Health Stroke Scale at admission (NIHSS, total score), admission ward (general ward, stroke unit, intensive-care unit, other ward), treatments performed in the hospital (systemic thrombolysis, intra-arterial thrombolysis or thrombectomy, ventilation), complications in the in-patient setting (increased intracranial pressure, symptomatic ICB), determination of a palliative treatment goal during hospitalization (yes vs. no), and the modified Rankin scale at discharge from hospital (0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability).
Outcome
The outcome on which the study focused was the frequency of hospital discharge of stroke patients to a free-standing hospice facility. This information was derived from the item “reason for discharge”. Only patients who had left the hospital alive could thus be included in the analysis.
Statistical analyses
Both descriptive and inferential statistics were investigated. Nominal variables are presented in absolute numbers and relative proportions plus the frequency of missing data. For continuous variables we calculated the mean and standard deviation. Percentages with 95% confidence intervals (CIs) were calculated to determine the prevalence of hospital discharge to an in-patient hospice. The number of cases of stroke (IS, ICB, SAB) at the time of hospital admission formed the basis for this comparison. The prevalence is presented with crude and age-standardized figures, using the “old European population” as the standard population. Tests included whether there were any significant differences among stroke patients in relation to the disease entity (ICB vs. IS; SAB vs. IS).
For continuous data, the unpaired t-test (for parametric data) or Mann-Whitney U test (for nonparametric data) were used; for categorical data, the chi-square test or Fisher’s exact test (frequency in cell <5) were used. The significance level was set at P<0.05 (two-sided). Because of multiple testing of the same population, Bonferroni correction was also performed to avoid alpha error accumulation. Factors influencing the target event of post-hospital hospice transfer were calculated using binary multivariate regression modeling. The variable selection was based on clinical considerations. The following were selected as influencing variables: sex (women vs. men), age (>80 or 60–80 vs. <60 years), disease entity (ICB or SAB vs. IS), prehospital care setting (care at home or care in an institution vs. independent at home), level of consciousness at admission (reduced vs. clear), extent of physical disability at discharge (modified Rankin scale: 5 or 4 or 3 vs. 0–2), and physician’s determination of a palliative treatment goal during hospitalization (yes vs. no). The quality of the statistical model was expressed using Nagelkerk’s pseudo-R2 coefficients. The software program IBM SPSS Statistics, version 28, was used for statistical analysis.
Ethics vote
A collaboration agreement was concluded with the Institute of Epidemiology and Social Medicine at the University of Münster, the coordinating center for the Northwest Germany Stroke Registry. The patient data are collected anonymized in the hospitals included in the quality assurance program. Therefore, a consultation with the ethics committee of the Medical Association of Westphalia-Lippe and the Medical Faculty of the Westphalian Wilhelms University of Münster was not necessary. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Selection of stroke patients documented in the registry
From 2017 to 2020, a total of 462,592 cases of adult patients with stroke were documented in the registry. From these, 339,513 cases with a principal diagnosis of IS, ICB, or SAB were included in the analysis. The frequency distribution of each diagnosis was as follows: IS 90.7% (n=308,067), ICB 7.9% (n=26,957), and SAB 1.3% (n=4,489) (Figure 1).
Characteristics of stroke patients
The mean age of the stroke patients was 73.1±13.1 years (women: 76.0 years, men: 70.5 years) (Table 1). There was a slight predominance of male patients, at 52.6%. The acute stroke had occurred less than 8 days before admission in 93.7% of cases (n=318,179); 77.1% of the patients (n=261,824) were treated in a stroke unit, and the medical objective was assessed as purely palliative during hospitalization in 10.4% of the cases. A total of 26,037 (7.7%) stroke patients died during their hospitalization.
Table 1
| Variables | IS (N=308,067) | ICB (N=26,957) | ICB vs. IS | SAB (N=4,489) | SAB vs. IS | All cases of stroke (N=339,513) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | N | % | N | P | % | N | P | % | N | ||||||
| Sex | |||||||||||||||
| Female | 47.1 | 145,106 | 47.3 | 12,738 | 0.647 | 57.8 | 2,597 | <0.001* | 47.3 | 160,441 | |||||
| Male | 52.8 | 162,763 | 52.6 | 14,204 | 0.647 | 42.1 | 1,888 | <0.001* | 52.6 | 178,927 | |||||
| n/a | 0.1 | 198 | 0.1 | 15 | – | 0.1 | 4 | – | 0.1 | 217 | |||||
| Age, years | |||||||||||||||
| Total, mean (SD) | 73.1 | (13.0) | 73.8 | (13.0) | 0.001* | 63.1 | (15.5) | <0.001* | 73.1 | (13.1) | |||||
| Female, mean (SD) | 76.1 | (12.9) | 76.5 | (12.5) | 0.198 | 63.3 | (15.5) | <0.001* | 76.0 | (13.0) | |||||
| Male, mean (SD) | 70.5 | (12.5) | 71.4 | (13.0) | <0.001* | 62.7 | (15.4) | <0.001* | 70.5 | (12.6) | |||||
| 18 to <60 | 16.1 | 49,700 | 15.7 | 4,247 | 0.106 | 44.8 | 2,011 | <0.001* | 16.5 | 55,958 | |||||
| 60 to <80 | 47.1 | 145,200 | 45.7 | 12,318 | <0.001* | 38.8 | 1,743 | <0.001* | 46.9 | 159,261 | |||||
| ≥80 | 368 | 113,167 | 386 | 10,392 | <0001* | 164 | 735 | <0.001* | 36.6 | 124,294 | |||||
| n/a | 0.0 | 0 | 0.0 | 0 | – | 0.0 | 0 | – | 0.0 | 0 | |||||
| Disease status | |||||||||||||||
| Event ≤7 days before | 95.0 | 292,371 | 84.5 | 22,764 | <0.001* | 67.8 | 3,044 | <0.001* | 93.7 | 318,179 | |||||
| Event >7 days before | 3.5 | 10,922 | 3.3 | 895 | 0.057 | 5.6 | 250 | <0.001* | 3.6 | 12,067 | |||||
| Other status | 1.5 | 4,774 | 12.2 | 3,298 | <0.001* | 26.6 | 1,195 | <0.001* | 2.7 | 9,267 | |||||
| Comorbidity | |||||||||||||||
| Arterial hypertension | 79.7 | 245,636 | 70.4 | 18,976 | <0.001* | 34.6 | 1,554 | <0.001* | 78.4 | 266,166 | |||||
| n/a | 6.6 | 20,240 | 19.5 | 5,262 | – | 45.5 | 2,043 | – | 8.1 | 27,545 | |||||
| Diabetes mellitus | 27.6 | 84,987 | 17.3 | 4,677 | <0.001* | 6.6 | 298 | <0.001* | 26.5 | 89,962 | |||||
| n/a | 6.6 | 20,436 | 19.6 | 5,273 | – | 45.6 | 2,045 | – | 8.2 | 27,754 | |||||
| Atrial fibrillation | 27.3 | 83,905 | 21.6 | 5,813 | <0.001* | 6.0 | 269 | <0.001* | 26.5 | 89,987 | |||||
| n/a | 6.9 | 21,363 | 19.7 | 5,312 | – | 45.6 | 2,049 | – | 8.5 | 28,724 | |||||
| Previous stroke | 24.1 | 74,197 | 17.8 | 4,801 | <0.001* | 7.0 | 313 | <0.001* | 23.4 | 79,311 | |||||
| n/a | 6.6 | 20,470 | 19.6 | 5,271 | – | 45.6 | 2,046 | – | 8.2 | 27,787 | |||||
| Prehospital care | |||||||||||||||
| Independent, at home | 75.6 | 232,904 | 63.7 | 17,179 | <0.001* | 61.0 | 2,738 | <0.001* | 74.5 | 252,821 | |||||
| Care at home | 10.7 | 33,004 | 11.5 | 3,094 | <0.001* | 4.4 | 197 | <0.001* | 10.7 | 36,295 | |||||
| Institutional care | 8.5 | 26,272 | 9.3 | 2,500 | <0.001* | 2.5 | 113 | <0.001* | 8.5 | 28,885 | |||||
| n/a | 5.2 | 15,887 | 15.5 | 4,184 | – | 32.1 | 1,441 | – | 6.3 | 21,512 | |||||
| Patient status at hospital admission | |||||||||||||||
| Consciousness | |||||||||||||||
| Clear awareness | 87.4 | 269,063 | 55.3 | 14,887 | <0.001* | 47.6 | 2,133 | <0.001* | 84.4 | 286,083 | |||||
| Reduced awareness | 7.6 | 23,461 | 29.3 | 7,878 | <0.001* | 20.5 | 920 | <0.001* | 9.5 | 32,259 | |||||
| n/a | 5.0 | 15,543 | 15.4 | 4,192 | – | 31.9 | 1,436 | – | 6.1 | 21,171 | |||||
| NIHSS score | |||||||||||||||
| 0 point | 10.4 | 32,079 | 4.6 | 1,250 | <0.001* | 26.9 | 1,206 | <0.001* | 10.2 | 34,535 | |||||
| 1–4 points | 43.8 | 134,946 | 20.9 | 5,635 | <0.001* | 18.4 | 827 | <0.001* | 41.7 | 141,408 | |||||
| 5–15 points | 31.0 | 95,597 | 33.0 | 8,885 | <0.001* | 10.4 | 467 | <0.001* | 30.9 | 104,949 | |||||
| 16–20 points | 5.8 | 17,761 | 10.9 | 2,928 | <0.001* | 3.0 | 134 | <0.001* | 6.1 | 20,823 | |||||
| 21–42 points | 3.8 | 11,683 | 15.0 | 4,031 | <0.001* | 9.2 | 411 | <0.001* | 4.7 | 16,125 | |||||
| n/a | 5.2 | 16,001 | 15.7 | 4,228 | – | 32.2 | 1,444 | – | 6.4 | 21,673 | |||||
| Mean (SD) | 5.9 | (6.4) | 11.6 | (9.8) | <0.001* | 6.7 | (10.5) | <0.001* | 6.4 | (6.9) | |||||
| In-patient treatment | |||||||||||||||
| Ward | |||||||||||||||
| General ward | 8.5 | 26,085 | 6.4 | 1717 | <0.001* | 8.1 | 365 | 0.433 | 8.3 | 28,167 | |||||
| Stroke unit | 79.5 | 244,840 | 57.7 | 15,550 | <0.001* | 32.0 | 1,434 | <0.001* | 77.1 | 261,824 | |||||
| Intensive-care unit | 6.0 | 18,349 | 19.7 | 5,323 | <0.001* | 26.6 | 1,196 | <0.001* | 7.3 | 24,868 | |||||
| Other ward | 0.2 | 621 | 0.5 | 147 | <0.001* | 1.0 | 46 | <0.001* | 0.2 | 814 | |||||
| n/a | 5.9 | 18,172 | 15.7 | 4,220 | – | 32.3 | 1,448 | – | 7.0 | 23,840 | |||||
| Treatment | |||||||||||||||
| Systemic lysis in hospital | 16.8 | 51,659 | 0.0 | 0 | – | 0.0 | 0 | – | 15.2 | 51,659 | |||||
| n/a | 4.9 | 15,245 | 15.4 | 4,152 | – | 31.9 | 1,431 | – | 6.1 | 20,828 | |||||
| Intra-art. lysis/thrombectomy | 3.1 | 9,677 | 0.0 | 0 | – | 0.0 | 0 | – | 2.9 | 9,677 | |||||
| n/a | 5.1 | 1,5642 | 15.5 | 4,187 | – | 32.2 | 1,444 | – | 6.3 | 21,273 | |||||
| Ventilation | 5.2 | 15,963 | 12.2 | 3,299 | <0.001* | 12.9 | 580 | <0.001* | 5.8 | 19,842 | |||||
| n/a | 5.0 | 15,299 | 15.4 | 4,160 | – | 31.9 | 1,433 | – | 6.2 | 20,892 | |||||
| Complications | |||||||||||||||
| Increased ICP | 1.6 | 4,777 | 10.1 | 2,735 | <0.001* | 6.6 | 296 | <0.001* | 2.3 | 7,808 | |||||
| n/a | 93.4 | 287,606 | 74.3 | 20,003 | – | 61.2 | 2,748 | – | 91.4 | 310,384 | |||||
| Secondary bleeding | 1.3 | 4126 | 4.8 | 1,290 | <0.001* | 2.8 | 126 | <0.001* | 1.6 | 5,542 | |||||
| n/a | 93.6 | 288,249 | 79.7 | 21,474 | – | 65.0 | 2,918 | – | 92.1 | 312,641 | |||||
| Modified Rankin scale at discharge | |||||||||||||||
| 0 | 16.1 | 49,481 | 4.2 | 1,144 | <0.001* | 14.4 | 648 | 0.003* | 15.1 | 5,1273 | |||||
| 1 | 21.1 | 64,933 | 8.1 | 2,182 | <0.001* | 11.6 | 521 | <0.001* | 19.9 | 67,636 | |||||
| 2 | 21.4 | 65,871 | 11.6 | 3,127 | <0.001* | 7.0 | 316 | <0.001* | 20.4 | 69,314 | |||||
| 3 | 13.0 | 40,131 | 10.8 | 2,907 | <0.001* | 4.8 | 214 | <0.001* | 12.7 | 43,252 | |||||
| 4 | 9.2 | 28,201 | 11.8 | 3,181 | <0.001* | 3.5 | 157 | <0.001* | 9.3 | 31,539 | |||||
| 5 | 7.1 | 21,758 | 13.8 | 3,707 | <0.001* | 6.5 | 293 | 0.169 | 7.6 | 25,758 | |||||
| Death | 6.0 | 18,623 | 25.3 | 6,818 | <0.001* | 13.3 | 596 | <0.001* | 7.7 | 26,037 | |||||
| n/a | 6.2 | 19,067 | 14.4 | 3,891 | 38.9 | 1,744 | 7.3 | 24,702 | |||||||
| Palliative treatment goal determined during hospital stay | |||||||||||||||
| Palliative treatment | 9.6 | 29,466 | 20.2 | 5,453 | <0.001* | 6.6 | 295 | <0.001* | 10.4 | 35,214 | |||||
| n/a | 6.8 | 21,071 | 19.7 | 5,309 | – | 45.9 | 2,059 | – | 8.4 | 28,439 | |||||
| Post-hospital transfer | |||||||||||||||
| Rehabilitation unit | 15.0 | 46,092 | 19.2 | 5,177 | <0.001* | 8.3 | 373 | <0.001* | 15.2 | 51,641 | |||||
| Other hospital | 9.1 | 28,082 | 13.4 | 3,619 | <0.001* | 23.3 | 1,048 | <0.001* | 9.6 | 32,749 | |||||
| Nursing home | 4.2 | 12,890 | 4.1 | 1,113 | 0.679 | 1.1 | 49 | <0.001* | 4.1 | 14,052 | |||||
| Hospice | 0.1 | 414 | 0.3 | 76 | <0.001* | 0.2 | 7 | 0.678 | 0.1 | 497 | |||||
| n/a | 5.2 | 15,965 | 15.6 | 4,209 | – | 32.2 | 1,447 | – | 6.4 | 21,621 | |||||
*, significant result according to Bonferroni correction. IS, ischemic stroke; ICB, intracerebral bleeding; SAB, subarachnoid bleeding; ICP, intracranial pressure; n/a, not available (data missing); SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale.
Patients with ICB or SAB had a more severe course of disease in comparison with patients with IS. This was reflected, among other things, in the frequency of the presence of reduced awareness at admission (ICB or SAB vs. IS; 29.3% or 20.5% vs. 7.6%, respectively; P<0.001) and in the fatality rate (ICB or SAB vs. IS; 25.3% or 13.3% vs. 6.0%, respectively; P<0.001). The mean age of patients with SAB was younger than in ICB and IS patients (SAB 63.1; ICB 73.8, IS 73.1 years). The proportion of women was also higher in SAB patients (57.8%) in comparison with ICB patients (47.3%) and IS patients (47.1%).
The documentation for the stroke registry was focused on acute care in hospital (Table S1). The time interval from event admission was ≤7 days in 93.7% of the cases (n=318,179); the event had occurred more than 7 days previously in 3.6% of cases (n=12,067); and other reasons for creating a MDS were reported in 2.7% of cases (n=9,267). The mean age of stroke patients with a recent event (≤7 days) was higher in comparison with patients with a longer interval (>7 days). With regard to the frequency of post-hospital hospice transfer, there were no differences between patients with events ≤7 vs. >7 days (0.1% vs. 0.1%; P=0.529). Analyses of cases in which the patients were discharged alive vs. deceased showed that deceased hospital patients were older on average, more likely to have cerebral hemorrhage, and more likely to have reduced awareness at admission, and that the majority of these patients had been classified as palliative cases by the physician (Table S2). Table S3 also provides a comparative overview of the characteristics of stroke patients with and without the determination of a palliative treatment goal during their hospital stay.
Frequency of hospital discharge to an in-patient hospice
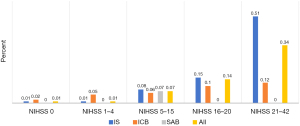
A total of 497 patients among 339,513 patients with stroke were discharged to free-standing hospices at the end of their in-patient care (Table 2). This represents a crude prevalence of 0.15% (95% CI: 0.13–0.16%) and an age-standardized prevalence of 0.05% (95% CI: 0.04–0.06%). Comparison of stroke entities showed that patients with ICB had a higher (age-standardized) prevalence of hospice transfer, at 0.07% (95% CI: 0.05–0.09%) than patients with IS, at 0.05% (95% CI: 0.04–0.06%) and SAB, at 0.01% (95% CI: 0.00–0.02%). A proportional relationship was observed between the severity of illness (NIHSS score) and increasing frequency of hospice transfer (Figure 2). In addition, a higher (age-standardized) percentage of patients with reduced awareness at hospital admission were transferred to in-patient hospices in comparison with patients without this clinical symptom (0.16% vs. 0.04%); the percentage was also higher in patients with a palliative treatment goal compared to those without (1.54% vs. 0.01%).
Table 2
| Variables | IS (N=308,067) | ICB (N=26,957) | ICB vs. IS | SAB (N=4,489) | SAB vs. IS | All stroke cases (N=339,513) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (95% CI) (%) | Transfer to hospice | Prevalence (95% CI) (%) | Transfer to hospice | P | Prevalence (95% CI) (%) | Transfer to hospice | P | Prevalence (95% CI) (%) | Transfer to hospice | ||||||
| Total | 414 | 76 | <0.001* | 7 | 0.678 | 497 | |||||||||
| Crude | 0.13 (0.12–0.15) | 0.28 (0.22–0.35) | 0.16 (0.04–0.27) | 0.15 (0.13–0.16) | |||||||||||
| Age-standardized | 0.05 (0.04–0.06) | 0.07 (0.05–0.09) | 0.01 (0.00–0.02) | 0.05 (0.04–0.06) | |||||||||||
| Gender | |||||||||||||||
| Women | N=145,106 | 264 | N=12,738 | 42 | <0.001* | N=2,597 | 5 | 0.815 | N=160,441 | 311 | |||||
| Crude | 0.18 (0.16–0.20) | 0.33 (0.23–0.43) | 0.19 (0.02–0.36) | 0.19 (0.17–0.22) | |||||||||||
| Age-standardized | 0.06 (0.04–0.08) | 0.09 (0.07–0.11) | 0.02 (0.01–0.03) | 0.06 (0.04–0.08) | |||||||||||
| Men | N=162,763 | 148 | N=14,204 | 34 | <0.001* | N=1,888 | 2 | 0.691 | N=178,855 | 184 | |||||
| Crude | 0.09 (0.08–0.11) | 0.24 (0.16–0.32) | 0.11 (0.00–0.25) | 0.10 (0.09–0.12) | |||||||||||
| Age-standardized | 0.04 (0.03–0.05) | 0.06 (0.04–0.08) | 0.00 (0.00–0.00) | 0.06 (0.04–0.08) | |||||||||||
| n/a | N=192 | 2 | N=15 | 0 | – | N=4 | 0 | – | N=211 | 2 | |||||
| Age | |||||||||||||||
| <60 years | N=49,700 | 18 | N=4,247 | 5 | 0.031 | N=2,011 | 0 | – | N=55,958 | 23 | |||||
| 0.04 (0.02–0.05) | 0.12 (0.01–0.22) | 0.00 (0.00–0.00) | 0.04 (0.02–0.06) | ||||||||||||
| 60 to <80 years | N=145,200 | 147 | N=12,318 | 27 | <0.001* | N=1,743 | 5 | 0.036 | N=159,261 | 179 | |||||
| 0.10 (0.08–0.12) | 0.22 (0.14–0.30) | 0.29 (0.04–0.54) | 0.11 (0.10–0.13) | ||||||||||||
| ≥80 years | N=113,167 | 249 | N=10,392 | 44 | <0.001* | N=735 | 2 | 0.679 | N=124,294 | 295 | |||||
| 0.22 (0.19–0.25) | 0.42 (0.30–0.55) | 0.27 (0.00–0.65) | 0.24 (0.21–0.26) | ||||||||||||
| n/a | N=133 | 0 | N=7 | 0 | – | N=1 | 0 | – | N=144 | 0 | |||||
| Prehospital care | |||||||||||||||
| Independent, at home | N=232,904 | 231 | N=17,179 | 30 | 0.005* | N=2,738 | 1 | 0.532 | N=252,821 | 262 | |||||
| Crude | 0.10 (0.09–0.11) | 0.17 (0.11–0.24) | 0.04 (0.00–0.11) | 0.10 (0.09–0.12) | |||||||||||
| Age-standardized | 0.03 (0.02–0.04) | 0.05 (0.04–0.06) | 0.01 (0.00–0.02) | 0.03 (0.02–0.04) | |||||||||||
| Care at home | N=33,004 | 96 | N=3,094 | 15 | 0.086 | N=197 | 0 | – | N=36,295 | 111 | |||||
| Crude | 0.29 (0.23–0.35) | 0.48 (0.24–0.73) | 0.00 (0.00–0.00) | 0.31 (0.25–0.36) | |||||||||||
| Age-standardized | 0.99 (0.93–1.05) | 0.19 (0.16–0.22) | 0.03 (0.02–0.04) | 0.83 (0.77–0.89) | |||||||||||
| Institutional care | N=26,272 | 63 | N=2,500 | 12 | 0.037 | N=113 | 0 | – | N=28,885 | 75 | |||||
| Crude | 0.24 (0.18–0.30) | 0.48 (0.21–0.75) | 0.21 (0.00–0.63) | 0.26 (0.20–0.32) | |||||||||||
| Age-standardized | 0.07 (0.05–0.09) | 0.22 (0.19–0.25) | 0.00 (0.00–0.00) | 0.09 (0.07–0.11) | |||||||||||
| n/a | N=15,887 | 24 | N=4,184 | 19 | – | N=1,441 | 6 | – | N=21,463 | 49 | |||||
| Awareness | |||||||||||||||
| Clear awareness | N=269,063 | 242 | N=14,887 | 27 | 0.987 | N=2,133 | 0 | – | N=286,083 | 269 | |||||
| Crude | 0.09 (0.08–0.10) | 0.18 (0.11–0.25) | 0.00 (0.00–0.00) | 0.09 (0.08–0.11) | |||||||||||
| Age-standardized | 0.04 (0.03–0.05) | 0.03 (0.02–0.04) | 0.00 (0.00–0.00) | 0.04 (0.03–0.05) | |||||||||||
| Reduced awareness | N=23,461 | 151 | N=7,878 | 31 | 0.009* | N=920 | 1 | – | N=32,259 | 183 | |||||
| Crude | 0.64 (0.54–0.75) | 0.39 (0.26–0.53) | 0.11 (0.00–0.32) | 0.57 (0.49–0.65) | |||||||||||
| Age-standardized | 0.18 (0.15–0.21) | 0.15 (0.13–0.17) | 0.04 (0.03–0.05) | 0.16 (0.14–0.18) | |||||||||||
| n/a | N=15,543 | 21 | N=4,192 | 18 | – | N=1,436 | 6 | – | N=21,171 | 45 | |||||
| Modified Rankin scale at hospital discharge | |||||||||||||||
| 0–2 | N=180,285 | 26 | N=6,453 | 1 | 0.613 | N=1,485 | 0 | – | N=188,223 | 27 | |||||
| Crude | 0.01 (0.01–0.02) | 0.00 (0.00–0.00) | 0.01 (0.01–0.02) | ||||||||||||
| Age-standardized | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ||||||||||||
| 3 | N=40,131 | 27 | N=2,907 | 1 | 0.999 | N=214 | 0 | – | N=43,252 | 28 | |||||
| Crude | 0.07 (0.04–0.09) | 0.00 (0.00–0.00) | 0.06 (0.04–0.09) | ||||||||||||
| Age-standardized | 0.03 (0.02–0.04) | 0.00 (0.00–0.00) | 0.02 (0.01–0.03) | ||||||||||||
| 4 | N=28,201 | 57 | N=3,181 | 6 | 0.999 | N=157 | 0 | – | N=31,539 | 63 | |||||
| Crude | 0.20 (0.15–0.25) | 0.19 (0.04–0.34) | 0.00 (0.00–0.00) | 0.20 (0.15–0.25) | |||||||||||
| Age-standardized | 0.09 (0.07–0.11) | 0.02 (0.01–0.03) | 0.00 (0.00–0.00) | 0.08 (0.06–0.10) | |||||||||||
| 5 | N=21,758 | 282 | N=3,707 | 50 | 0.761 | N=293 | 1 | 0.192 | N=25,758 | 333 | |||||
| Crude | 1.30 (1.15–1.45) | 1.35 (0.98–1.72) | 0.34 (0.00–1.01) | 1.29 (1.15–1.43) | |||||||||||
| Age-standardized | 0.82 (0.76–0.88) | 0.38 (0.28–0.36) | 0.13 (0.11–0.15) | 0.69 (0.64–0.74) | |||||||||||
| n/a | N=19,067 | 22 | N=3,891 | 18 | – | N=1,744 | 6 | – | N=24,702 | 46 | |||||
| Palliative treatment goal determined during hospital stay | |||||||||||||||
| No | N=278,601 | 111 | N=21,504 | 14 | – | N=4,194 | 0 | – | N=304,299 | 125 | |||||
| Crude | 0.04 (0.03–0.05) | 0.07 (0.03–0.10) | 0.00 (0.00–0.00) | 0.04 (0.03–0.05) | |||||||||||
| Age-standardized | 0.01 (0.00–0.02) | 0.02 (0.00–0.05) | 0.00 (0.00–0.00) | 0.01 (0.00–0.02) | |||||||||||
| Yes | N=29,466 | 281 | N=5,453 | 44 | 0.319 | N=295 | 1 | 0.535 | N=35,214 | 326 | |||||
| Crude | 0.95 (0.84–1.06) | 0.81 (0.57–1.04) | 0.34 (0.00–1.00) | 0.93 (0.83–1.03) | |||||||||||
| Age-standardized | 1.93 (1.66–2.20) | 0.53 (0.39–0.67) | 0.09 (0.03–0.15) | 1.54 (1.30–1.78) | |||||||||||
| n/a | N=21,071 | 22 | N=5,309 | 18 | – | N=2,059 | 6 | – | N=28,439 | 46 | |||||
*, significant result according to Bonferroni correction. n/a, not available (data missing); CI, confidence interval; IS, ischemic stroke; ICB, intracerebral bleeding; SAB, subarachnoid bleeding.
Characteristics of stroke patients with hospice transfer vs. those without
In-patients with stroke who were discharged to hospices had a higher mean age (80.0 vs. 72.5 years; P<0.001), included a higher percentage of women (62.6% vs. 47.2%; P<0.001) and a higher proportion of ICB patients (15.3% vs. 7.9%; P<0.001), and more often had reduced awareness at hospital admission (36.8% vs. 9.5%; P<0.001) (Table 3). In addition, they were significantly more likely to be suffering from severe disability at the time of hospital discharge (modified Rankin scale grade 5: 67.0% vs. 7.5%; P<0.001).
Table 3
| Variables | Transferred to a hospice (N=497) | Not transferred to a hospice (N=339,016) | P | |||
|---|---|---|---|---|---|---|
| % | N | % | N | |||
| Sex | ||||||
| Female | 62.6 | 311 | 47.2 | 160,130 | <0.001* | |
| Male | 37.0 | 184 | 52.7 | 178,671 | <0.001* | |
| n/a | 0.4 | 2 | 0.1 | 215 | – | |
| Age, years | ||||||
| Total, mean (SD) | 80.0 | (10.6) | 72.5 | (13.1) | <0.001* | |
| Female, mean (SD) | 81.3 | (10.3) | 75.3 | (13.1) | <0.001* | |
| Male, mean (SD) | 77.7 | (10.9) | 70.0 | (12.5) | <0.001* | |
| <60 | 4.6 | 23 | 16.5 | 55,935 | <0.001* | |
| 60 to <80 | 36.0 | 179 | 46.9 | 159,082 | <0.001* | |
| ≥80 | 59.4 | 295 | 36.6 | 123,999 | <0.001* | |
| n/a | 0.0 | 0 | 0.0 | 0 | – | |
| Disease status | ||||||
| Event ≤7 days before | 90.9 | 452 | 93.7 | 317,727 | 0.013 | |
| Event >7 days before | 2.8 | 14 | 3.6 | 12,053 | 0.461 | |
| Other status | 6.2 | 31 | 2.7 | 9,236 | – | |
| Type of stroke | ||||||
| Ischemic stroke | 83.3 | 414 | 90.7 | 307,653 | <0.001* | |
| Intracerebral bleeding | 15.3 | 76 | 7.9 | 26,881 | <0.001* | |
| Subarachnoid bleeding | 1.4 | 7 | 1.3 | 4,482 | 0.842 | |
| Comorbidity | ||||||
| Arterial hypertension | 77.7 | 386 | 78.4 | 265,780 | 0.706 | |
| n/a | 9.3 | 46 | 8.1 | 27,499 | – | |
| Diabetes mellitus | 24.5 | 122 | 26.5 | 89,840 | 0.334 | |
| n/a | 9.3 | 46 | 8.2 | 27,708 | – | |
| Atrial fibrillation | 39.5 | 196 | 26.4 | 89,791 | <0.001* | |
| n/a | 9.7 | 48 | 8.5 | 28,676 | – | |
| Previous stroke | 27.4 | 136 | 23.4 | 79,175 | 0.037 | |
| n/a | 9.3 | 46 | 8.2 | 27,741 | – | |
| Prehospital care | ||||||
| Independent, at home | 52.7 | 262 | 74.5 | 252,669 | <0.001* | |
| Care at home | 22.3 | 111 | 10.7 | 36,198 | <0.001* | |
| Institutional care | 15.1 | 75 | 8.5 | 28,822 | <0.001* | |
| n/a | 9.9 | 49 | 6.3 | 21,485 | – | |
| Patient status at hospital admission | ||||||
| Consciousness | ||||||
| Clear awareness | 54.1 | 269 | 84.3 | 285,814 | <0.001* | |
| Reduced awareness | 36.8 | 183 | 9.5 | 32,076 | <0.001* | |
| n/a | 9.1 | 45 | 6.2 | 21,126 | – | |
| NIHSS score | ||||||
| 0 point | 2.0 | 10 | 10.2 | 34,525 | <0.001* | |
| 1–4 points | 10.9 | 54 | 41.7 | 141,354 | <0.001* | |
| 5–15 points | 39.2 | 195 | 30.9 | 104,754 | <0.001* | |
| 16–20 points | 20.1 | 100 | 6.1 | 20,723 | <0.001* | |
| 21–42 points | 17.9 | 89 | 4.7 | 16,036 | <0.001* | |
| n/a | 9.9 | 49 | 6.4 | 21,624 | – | |
| Mean (SD) | 13.8 | (8.0) | 6.4 | (6.7) | <0.001* | |
| In-patient treatment | ||||||
| Ward | ||||||
| General ward | 7.0 | 35 | 8.3 | 28,132 | 0.369 | |
| Stroke unit | 70.6 | 351 | 77.1 | 261,473 | <0.001* | |
| Intensive-care unit | 12.7 | 63 | 7.3 | 24,805 | <0.001* | |
| Other ward | 0.2 | 1 | 0.2 | 813 | 0.999 | |
| n/a | 9.5 | 47 | 7.0 | 23,793 | – | |
| Treatment | ||||||
| Systemic lysis in hospital | 10.9 | 54 | 15.2 | 51,605 | 0.007* | |
| n/a | 9.1 | 45 | 6.1 | 20,783 | – | |
| Intra-arterial lysis/thrombectomy | 7.0 | 35 | 2.8 | 9,642 | <0.001* | |
| n/a | 9.1 | 45 | 6.3 | 21,228 | – | |
| Ventilation | 10.5 | 52 | 5.8 | 19,790 | <0.001* | |
| n/a | 9.1 | 45 | 6.1 | 20,847 | – | |
| Complications | ||||||
| Increased intracranial pressure | 9.3 | 46 | 2.3 | 7,762 | <0.001* | |
| n/a | 81.7 | 406 | 91.4 | 309,978 | – | |
| Secondary bleeding | 5.4 | 27 | 1.6 | 5,515 | <0.001* | |
| n/a | 85.5 | 425 | 92.1 | 312,216 | – | |
| Modified Rankin scale at discharge | ||||||
| 0 | 0.8 | 4 | 15.1 | 51,269 | <0.001* | |
| 1 | 1.4 | 7 | 19.9 | 67,629 | <0.001* | |
| 2 | 3.2 | 16 | 20.4 | 69,298 | <0.001* | |
| 3 | 5.6 | 28 | 12.8 | 43,224 | <0.001* | |
| 4 | 12.7 | 63 | 9.3 | 31,476 | 0.011 | |
| 5 | 67.0 | 333 | 7.5 | 25,425 | <0.001* | |
| Death | 0.0 | 0 | 7.7 | 26,037 | – | |
| n/a | 9.3 | 46 | 7.3 | 24,656 | – | |
| Palliative treatment goal determined during hospital stay | ||||||
| Palliative treatment | 65.6 | 326 | 10.3 | 34,888 | <0.001* | |
| n/a | 9.3 | 46 | 8.4 | 28,393 | – | |
*, significant result according to Bonferroni correction. n/a, not available (data missing); SD, standard deviation; NIHSS, National Institute of Health Stroke scale.
Factors influencing hospice discharge
In general, the chance of hospice transfer increased with the extent of physical impairment due to stroke (Table 4). The odds of hospice transfer increased by a factor of 3.54 for patients with moderate disability at the time of hospital discharge (Rankin scale grade 3) in comparison with patients with no symptoms or slight disability (grades 0–2), by a factor of 9.06 for higher-grade disability (grade 4), and by a factor of 34.78 for severe disability (grade 5). The physician’s determination of a palliative treatment goal during the hospital stay increased the odds of a hospice transfer by a factor of 14.22 compared with patients without a palliative approach. If patients had reduced awareness at hospital admission, the probability of hospice transfer increased by a factor of 1.71 (OR 1.71; 95% CI: 1.39–2.10; P<0.001). Patients who had been cared for in a nursing home before admission to hospital were less likely to be transferred to an in-patient hospice after their hospital stay in comparison with patients who were independent at home (OR 0.34; 95% CI: 0.25–0.44; P<0.001).
Table 4
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Women [1] vs. men [0] | 1.13 | 0.92–1.39 | 0.252 |
| Age, years | |||
| 60 to <80 [1] vs. <60 [0] | 1.33 | 0.83–2.13 | 0.244 |
| ≥80 [1] vs. <60 [0] | 1.39 | 0.86–2.24 | 0.179 |
| Stroke entity | |||
| IS [1] vs. ICB [0] | 0.95 | 0.71–1.27 | 0.722 |
| SAB [1] vs. ICB [0] | 0.28 | 0.04–2.02 | 0.207 |
| Prehospital care | |||
| Care at home [1] vs. independent at home [0] | 0.91 | 0.72–1.15 | 0.441 |
| Institutional care [1] vs. independent at home [0] | 0.34 | 0.25–0.44 | <0.001 |
| Consciousness | |||
| Reduced [1] vs. clear awareness [0] | 1.71 | 1.39–2.10 | <0.001 |
| Modified Rankin scale at discharge | |||
| 3 [1] vs. 0–2 [0] | 3.54 | 2.08–6.03 | <0.001 |
| 4 [1] vs. 0–2 [0] | 9.06 | 5.72–14.36 | <0.001 |
| 5 [1] vs. 0–2 [0] | 34.78 | 22.94–52.75 | <0.001 |
| Palliative treatment goal determined during hospital stay | |||
| Yes [1] vs. no [0] | 14.22 | 11.32–17.87 | <0.001 |
| Goodness of the statistical model | |||
| Nagelkerk R2 | 0.298 | ||
[1] factor of influence; [0] reference factor. CI, confidence intervals; ICB, intracerebral bleeding; IS, ischemic stroke; OR, odds ratio; SAB, subarachnoid bleeding.
Discussion
The present study, based on data from the Northwest-German Stroke Registry, indicates that in Germany, only a very small proportion of hospitalized patients with stroke are discharged to free-standing hospices at the end of their hospital stay. This was observed only in 0.05% of all in-patient stroke cases (age-standardized). Age-standardized prevalence was 0.05% for IS, 0.07% for ICB, and 0.01% for SAB.
These results stand in contrast to those of comparable studies, mainly in the United States, which have reported higher hospice transfer rates. For clearer assessment of the literature, it should be pointed out that in the United States, the term “hospice” is defined more broadly than in Germany. The term is associated there with hospice home care programs, independent hospice companies with or without in-patient facilities, palliative care departments in a hospital or university, and also palliative care teams that are affiliated to acute care hospitals. In Germany, hospices represent structurally, organizationally, and economically independent free-standing facilities with separate staff and a distinct approach. This distinguishes them from palliative wards, which are units integrated into the hospital. In Germany, there are approximately 250 in-patient hospices (2,550 beds) and approximately 340 palliative care units (2,784 beds) (as of February 2022). The European Association for Palliative Care (EAPC) has called for a standard of 80–100 hospice or palliative-care beds per 1 million population (42). The current actual figures are 30.6 hospice beds and 33.5 palliative-care beds per 1 million population (as of February 2022). The EAPC minimum standard has thus not yet been achieved in Germany. In everyday clinical practice, it is not uncommon for it to take several weeks before a hospice place can be offered after registration.
Comparative studies from the United States show higher rates of hospice transfer of in-patient stroke patients. In a single-center study as part of the “Get with The Guidelines Stroke Study”, Chauhan et al. examined 2,446 patients with IS who were hospitalized at the University of Arkansas from 2009 to 2015 (38). The methodology used was very similar to that in the present study. It was reported that 100 stroke patients (4.1%) were transferred to a hospice after their hospital stay. duPreez et al. evaluated United States insurance data (Medicare data) for claimants aged 64 years and older who were hospitalized for IS in 2000 and died within 30 days of the event (39). The focus was thus on cerebral infarction patients with a severe disease course. A total of 4,894 patients were identified, among whom nearly one in 4 (23.4%) were transferred to a hospice after discharge. Almost half of all patients who were able to leave the hospital alive (44.0%) were transferred to a hospice.
Frequency of stroke patients cared for in hospices
According to the National Hospice and Palliative Care Organization (NHPCO), just over 1 million people in the United States die in hospices each year, including 29.6% with a diagnosis of malignant tumor disease, 17.4% with cardiovascular disease, and 15.6% with dementia (43). At a frequency of 9.5%, stroke patients occupy sixth place in the ranking of the most frequent diagnoses among hospice patients (as of 2018). Unfortunately, no official statistics are available in Germany on the types of patients in free-standing hospices. Data from the National Hospice and Palliative Registry (NHPR), which are only accessible to registry participants, show that it is predominantly (80.6%) oncology patients who are cared for in hospices (as of 2019) (44).
Barriers to hospice transfer
One reason why stroke patients are rarely transferred to a hospice is the acute course of the disease. Studies on the topic of case fatalities show that despite medical advances in the diagnosis and treatment of stroke patients, approximately one in five to six patients die within the first 30 days after the acute event (5,45). The type of stroke strongly influences the risk of death in these cases, with cerebral hemorrhage being associated with a significantly higher risk of death in comparison with cerebral infarction. Data for the 30-day mortality thus range from 5.7% to 14.9% in patients with IS (4-9), from 29.6% to 46.5% in patients with intracerebral hemorrhage (5,10-12), and from 19.1% to 33.0% in patients with subarachnoid hemorrhage (5,13,14). A significant proportion of patients with stroke thus die in hospital. It is therefore common during in-patient care for stroke patients who are receiving best supportive care to be registered for a hospice, but to die in hospital while waiting for hospice placement. Unfortunately, there are no data on this in the literature.
Another reason for this is insufficient awareness of stroke as a “palliative condition” (26,34,35,45,46). Traditionally, specialized palliative care has mainly been reserved for patients with advanced-stage cancer, with only a small proportion of non-cancer patients (47,48). For example, analyses of a national hospice and palliative care survey in Germany show that the proportion of non-cancer patients cared for is less than 10% (48). For hospitalized stroke patients, this leads to various problems: firstly, patients are not placed in contact with a collaborating palliative care team at an early stage in the hospital. For example, data from a multicenter study including four major United States hospitals show that palliative consultants became involved for stroke patients with severe disease a median of 2–9 days before the patients’ deaths (37). Only 19.7% of the affected patients received a consultation from the palliative care service. This mainly affected critically ill patients. These research results are confirmed by other studies (49-51). Secondly, patients who are to be transferred from hospital to home or to a nursing home are not placed in contact with an outpatient specialized palliative care team. Thirdly, hospital physicians do not apply for hospice places for stroke patients at all, or only rarely. When specialized palliative care in a hospice is compared with in-patient nursing homes in Germany, clear advantages in favor of hospice transfer emerge. Hospices generally have a higher staffing ratio, a higher proportion of specialists trained in palliative care, regular involvement of trained palliative care physicians in patient care, and smaller numbers of beds (average 8–16 beds). In addition, unlike nursing homes, their funding is almost entirely covered by health insurance and long-term care insurance. Hospices are therefore also financially attractive. The German parliament recognized the shortcomings of palliative care in long-term in-patient care facilities and attempted to counteract it by passing the Hospice and Palliative Care Act [2015] (52). Among other things, this law makes it mandatory for nursing homes to enter into cooperation agreements with local providers of specialized palliative care. To date, however, national implementation of such obligatory agreements has not been achieved.
The transfer of nursing home patients to a hospice is problematic in everyday practice. Health-insurance companies do not generally recognize the need for hospice care for nursing home residents. They assume that nursing homes can provide comprehensive, palliative end-of-life care themselves, even for seriously ill patients who are approaching death. Although it is possible to circumvent this restrictive regulation by remaining in a palliative ward, transfers from nursing home to hospice continue to be difficult. The present study data confirm the problem. For example, nursing home residents with stroke who were discharged after a hospital stay were transferred back to the nursing home in 33.4% of cases, and only 0.3% were transferred to a hospice. The corresponding percentages were 42.5% and 1.1%, respectively, for stroke patients for whom a palliative treatment goal was established while they were still hospitalized.
To improve palliative care for stroke patients and their families in hospital, the following suggestions might be considered: (I) establishing joint physician rounds involving a palliative care physician or a palliative care nurse in stroke units and neurological/neurosurgical intensive-care units; (II) initiating interdisciplinary case conferences to clarify treatment goals and further care for critically ill stroke patients; (III) providing further training courses for hospital physicians and nurses to intensify their knowledge of specifically palliative medical and communicative content; and (IV) developing palliative wards and specialized palliative services in hospitals. Data from the German hospital system indicate that there is a structural deficit here. At the beginning of 2022, for example, only 18.3% of all hospitals included a palliative care unit. Only 3.8% had implemented an in-patient palliative service. This deficit in care provision is particularly unfortunate, as it has been demonstrated that including palliative care expertise in in-patient care leads to improved symptom control and increased quality of life for patients and their families (53,54). Some evidence is available that this leads to cost savings in health care, but the findings are not consistent across all of the studies (55).
Neurological factors influencing hospice transfer
The extent of neurological impairment due to stroke is a major determinant of hospice transfer. For example, a positive association has been demonstrated between increasing NIHSS scores and increasing frequency of hospice transfer. Among patients in Germany, the NIHSS score differed only slightly from the data in a controlled study in the United States (38). The presence of impaired consciousness was also associated with hospice transfer. If the patient’s awareness was reduced at the time of hospital admission, the chance of hospice transfer increased by a factor of 1.7. These results are not surprising, as they demonstrate a correct medical indication for hospice patients, whose life expectancy should be less than 6 months.
Strengths and limitations
The strength of the present study is the size of the data set (n=339,513) and the consistent collection of data over time. A limitation that should be noted is that the registry data mainly relate to patients who had a stroke event less than 8 days before hospital admission, so that the study primarily reflects cases of acute in-patient care. However, we intentionally included patients in whom the event occurred more than 7 days previously and/or in whom a palliative treatment goal had already been established at the time of hospital admission. Only a MDS was created for these patients, but it explicitly included discharge status. For all the patients in the stroke registry, it was therefore possible for the target event “hospice transfer” to be documented. If only a MDS was created, information on diagnostic investigation and treatment was missing, among other things, so that many items had a large proportion of missing values.
Participation in the stroke registry was voluntary for the hospitals involved. However, it was mainly hospitals with a stroke unit that took part, as this was mandatory for them in obtaining certification. The selection of participating hospitals can therefore not be considered representative for Germany.
It was not possible to identify some important factors that affect hospice transfer from the registry data. These include co-morbidities (e.g., malignant tumor, pneumonia, sepsis, decubitus ulcer), marital status, current life circumstances, and the availability of an advance health-care directive (living will) stating the patient’s wishes regarding performance or omission of life-prolonging measures (do not resuscitate/do not intubate status). Accordingly, the multivariate logistic regression model had limitations relative to the available influencing variables.
Conclusions
In Germany, only a very small percentage of adult stroke patients with a severe disease course are discharged to in-patient hospices at the end of their hospital stays. Although a not insignificant proportion of in-patients with stroke have exclusively palliative care needs, these patients are very rarely discharged to a free-standing hospice after hospitalization. Closer cooperation between various medical disciplines (e.g., neurology, neurosurgery, intensive care) and palliative medicine as well as the involvement of spiritual care (e.g., the hospital chaplaincy) would be desirable in order to contribute to improved, holistic care for seriously ill patients and their relatives at the end of life. It should also be critically called into question whether excluding from hospices those patients who have been receiving long-term nursing-home care—the usual practice in Germany—should continue.
Acknowledgments
The continuous commitment and effort to document stroke patients in all the centers participating in the Northwest Germany Stroke Registry is highly appreciated and acknowledged. The following centers contributed to the register: AMEOS Diakonie Clinic, Ueckermünde; AMEOS Clinical Center Bernburg, Bernburg; Agaplesion Bethesda Hospital Wuppertal, Wuppertal; Agaplesion Diakonieklinikum Rotenburg gGmbH, Rotenburg; Agnes-Karll-Hospital Laatzen, Laatzen; Celle General Hospital, Celle; Altmark Clinic Gardelegen, Gardelegen; Ameos Clinic Haldensleben, Haldensleben; Ammerland Klinik GmbH, Westerstede; Asklepios Fachklinikum Brandenburg, Brandenburg; Asklepios Lübben Specialist Hospital, Lübben; Asklepios Fachklinikum Stadtroda GmbH, Stadtroda; Asklepios Specialist Hospital Teupitz, Teupitz; Asklepios Clinic Pasewalk, Pasewalk; Asklepios Clinic Weissenfels, Weissenfels; Asklepios Uckermark Hospital, Schwedt; Asklepios Clinics Schildautal, Seesen; BDH Clinic Hessisch Oldendorf gGmbH, Hessisch Oldendorf; BG University Hospital Bergmannsheil GmbH, Bochum; BG Clinics Bergmannstrost, Halle (Saale); Cariatasklinikum Saarbrücken, Saarbrücken; Carl von Basedow KlinikumSaalekreis gGmbH, Merseburg; Carl Thiem Hospital, Cottbus; Centre Hospitalier Emile Mayrisch, ESCH-SUR-ALZETTE; Centre Hospitalier de Luxembourg, LUXEMBOURG; Melle Christian Hospital, Melle; Christian Hospital, Quakenbrück; Christophorus-Kliniken GmbH, Dülmen; Clemenshospital, Münster; DIAKOVERE Friederikenstift, Hanover; DIAKOVERE Henriettenstift, Hanover; DRK Hospital Luckenwalde, Luckenwalde; DRK Hospital Saarlouis, Saarlouis; DRK Manniske Hospital, Bad Frankenhausen; Diakonie Klinikum Neunkirchen gGmbH, Neunkirchen/Saar; Diakoniekrankenhaus Chemnitzer LandDIAKOMED gGmbH, Hartmannsdorf; Dietrich Bonhoeffer Clinic, Neubrandenburg; Eichsfeld Klinikum GmbH, Kleinbartloff OT Reifenstein; Elbe-Klinikum Stade, Stade; Elblandkliniken Meissen, Meissen; Elisabeth Hospital, Recklinghausen; Euregio Clinic, Nordhorn; Ev. Bathildiskrankenhaus Bad Pyrmont gGmbH, Bad Pyrmont; Ev. Hospital Bielefeld Johannesstift, Bielefeld; Ev. Krankenhaus Bielefeld gGmbH - Gilead I, Bielefeld; Ev. Hospital, Lippstadt; Evangelisches Krankenhaus GmbH, Gelsenkirchen; Ludwigsfelde-Teltow Protestant Hospital, Ludwigsfelde; Oldenburg Protestant Hospital, Oldenburg; Unna Protestant Hospital, Unna; Protestant Hospital, Castrop-Rauxel; Protestant Hospital, Hattingen; Protestant Hospital, Herne; Specialist Hospital Hubertusburg gGmbH, Wermsdorf; Herdecke Community Hospital, Herdecke; Gesundheit Nord – Klinikum Bremen Nord, Bremen; Health Center Bitterfeld/Wolfen, academic teaching hospital of Martin Luther University of Halle-Wittenberg, Bitterfeld-Wolfen; HELIOS Gifthorn Clinic, Gifthorn; HELIOS Vogtland Clinic Plauen, Plauen; Hans Susemihl Hospital, Emden; Hanse-Klinikum Stralsund, Stralsund; Hanse-Klinikum Wismar GmbH, Wismar; Havelland Kliniken GmbH, Nauen Clinic, Nauen; Havelland Kliniken GmbH, Rathenow site, Rathenow; Heidekreis-Klinikum GmbH, Soltau; Heinrich Braun Hospital Zwickau, Zwickau; Helios Clinic Lengerich, Lengerich; Helios Clinics Aue, Aue; Helios Clinics Schwerin, Schwerin; Helios Clinic Bad Saarow, Bad Saarow; Helios Clinic Erfurt, Erfurt; Helios Klinikum Hildesheim GmbH, Hildesheim; Helios Clinic Wuppertal-Barmen, Wuppertal; Helios Albert Schweitzer Hospital Northeim, Northeim; Helios District Hospital Gotha-Ohrdruf, Gotha; Sacred Heart Hospital, Münster-Hiltrup; Hufeland Klinikum GmbH, Mühlhausen; Hôpital St-Louis – Neurology, Ettelbruck; Ilm-Kreis-Kliniken Arnstadt-Ilmenau gGmbH, Arnstadt; Ilm-Kreis-Kliniken Arnstadt-Ilmenau gGmbH, Ilmenau; Immanuel Clinic Rüdersdorf, Rüdersdorf; Johannes Wesling Clinical Center Minden, Minden; KKRN Katholisches Klinikum Ruhrgebiet Nord GmbH, Marl; KMG Klinikum Güstrow GmbH, Güstrow; KMG Hospital Kyritz, Kyritz; KRH Klinikum Neustadt am Rübenberge, Neustadt am Rübenberge; KRH Klinikum Nordstadt, Hanover; KRH Robert Koch Gehrden Clinic, Gehrden; Catholic Hospital St. Johann Nepomuk, Erfurt; Erlabrunn gGmbH Clinics, Breitenbrunn; Maria Hilf Clinics, Mönchengladbach; Klinikum Altenburger Land GmbH, Altenburg; Klinikum Arnsberg GmbH, St. Johannes Hospital, Arnsberg; Klinikum Bad Salzungen GmbH, Bad Salzungen; Klinikum Barnim GmbH, Werner Forssmann KH, Eberswalde; Braunschweig Hospital, Braunschweig; Bremen-Mitte Clinic, Bremen; Bremerhaven-Reinkenheide Clinic, Bremerhaven; Chemnitz Hospital gGmbH, Chemnitz; Dorothea Christiane Erxleben GmbH Clinic, Wernigerode; Klinikum Frankfurt (Oder) GmbH, Frankfurt (Oder); Grossburgwedel Clinic, Burgwedel; Herford Hospital, Herford; Ibbenbüren Hospital, Ibbenbüren; Lippe-Lemgo Clinic, Lemgo; Lüdenscheid Hospital, Lüdenscheid; Magdeburg Hospital gGmbH, Magdeburg; Klinikum Meiningen GmbH, Meiningen; Klinikum Niederlausitz GmbH, Senftenberg; Osnabrück Hospital, Osnabrück; Peine Clinic, Peine; Saarbrücken Hospital gGmbH, Saarbrücken; Klinikum St. Georg gGmbH, Leipzig; Klinikum Stadt Soest, Soest; Uelzen Clinic, Uelzen; Klinikum Westfalen GmbH, Knappschaftskrankenhaus, Dortmund; Wolfsburg Hospital, Wolfsburg; Knappschaftskrankenhaus Püttlingen, Püttlingen; Miners’ Hospital, Bottrop; Miners’ Hospital, Recklinghausen; Miners’ Hospital, Sulzbach; Hospital Märkisch-Oderland GmbH, Strausberg; Hospital Plau am See, Plau am See; Hospital St. Elisabeth-Stift, Damme; Siegen District Hospital, Siegen; Freiberg District Hospital gGmbH, Freiberg; Greiz District Hospital GmbH, Greiz; Gummersbach District Hospital, Gummersbach; Prenzlau District Hospital, Prenzlau; Kreiskrankenhaus Prignitz gemeinnützige GmbH, Perleberg; Rudolf Virchow District Hospital, Glauchau; Schleiz District Hospital, Schleiz; LWL Clinic Lengerich, Lengerich; Ludmillenstift, Meppen; Marienhaus Clinic Saarlouis-Dillingen, Dillingen; Marienhospital Letmathe, Iserlohn; Marienhospital Osnabrück, Osnabrück; Marienkrankenhaus St. Wendel, St. Wendel; Martha-Maria Krankenhaus Halle-Dölau gGmbH, Halle (Saale); Martin Gropius Krankenhaus GmbH, Eberswalde; Martin Luther University Halle-Wittenberg, Halle (Saale); Medical School, Hanover; Mittelweser Kliniken GmbH, Nienburg; Muldentalkliniken GmbHHospital Wurzen, Wurzen; Northwest Hospital Sanderbusch, Sande; Oberhavel Kliniken GmbH - Hennigsdorf Clinic, Hennigsdorf; Otto von Guericke University Magdeburg, Magdeburg; Paracelsus Clinic Osnabrück, Osnabrück; Paracelsus Clinic Zwickau, Zwickau; Rhein-Maas Klinikum GmbH - Marienhöhe operating unit, Würselen; Robert-Koch-Krankenhaus-Apolda GmbH, Apolda; Ruppiner Kliniken GmbH, Neuruppin; SHG Merzig Clinic, Merzig; SRH Krankenhaus Waltershausen-Friedrichroda GmbH, Friedrichroda; SRH Waldklinikum Gera, Gera; SRH Central Hospital, Suhl; Saale-Unstrut-Klinikum Naumburg, Naumburg; Sana Clinics, Duisburg; Sana Clinics, Lübeck; Sana Klinikum Borna, Borna; Sana Hospital Templin, Templin; Sana Hospital Rügen GmbH, Bergen on Rügen; Sofien- und Hufeland-Klinikum GmbH, Weimar; St. Ansgar Hospital, Höxter; St. Barbara Hospital, Gladbeck; St. Bernward Hospital, Hildesheim; St. Elisabeth Hospital, Gütersloh; St. Franziskus Hospital, Ahlen; St. Georg Hospital Eisenach, Eisenach; St. Johannes Hospital, Hagen; St. Josef Hospital, Bochum; St. Marien-Hospital GmbH, Lünen; St. Mary’s Hospital, Borken; St. Mary’s Hospital, Hamm; St. Vincenz Hospital Paderborn, Paderborn; St. Vincenz Hospital, Menden; Municipal Clinics, Dortmund; Dresden Municipal Hospital, Friedrichstadt site, Dresden; Dresden Municipal Hospital - Neustadt site, Dresden; Görlitz Municipal Hospital, Görlitz; Wolfenbüttel Municipal Hospital gGmbH, Wolfenbüttel; Municipal Hospital, Lüneburg; Saxon Hospital Altscherbitz, Schkeuditz; Saxon Hospital Arnsdorf, Arnsdorf; Saxon Hospital, Rodewisch; Südharz-Krankenhaus Nordhausen GmbH, Nordhausen; Thuringia Clinics “Georgius Agricola”, Rudolstadt; UKSH, Campus Kiel, Kiel; University of Leipzig, Leipzig; University of Münster, Hier; University of Rostock, Rostock; University Hospital Aachen, Aachen; University Hospital Carl Gustav Carus Dresden, Dresden; University Hospital Göttingen, Göttingen; University Hospital Jena, Jena; University Hospital Knappschaftskrankenhaus Bochum, Bochum-Langendreer; Saarland University Hospital, Homburg/Saar; Greifswald University Medical Center, Greifswald; Waldkrankenhaus “Rudolf Elle” GmbH, Eisenberg; Zentralklinik Bad Berka GmbH, Bad Berka; ZithaKlinik S.A., Luxembourg; Ökumenisches Hainich Klinikum GmbH, Mühlhausen.
Funding: The authors acknowledge support from the Open Access Publication Fund of the University of Münster.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-418/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-418/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-418/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-418/coif). KB reports that the Northwest German Stroke Registry was financially supported by the German Ministry of Education and Research from 2003 to 2009. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient data are collected anonymized in the hospitals included in the quality assurance program. Therefore, a consultation with the ethics committee of the Medical Association of Westphalia-Lippe and the Medical Faculty of the Westphalian Wilhelms University of Münster was not necessary. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254-743. [Crossref] [PubMed]
- World Health Organization (WHO) (2020). The top 10 causes of death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed May 25, 2022.
- Belleudi V, Sciattella P, Agabiti N, et al. Socioeconomic differences in one-year survival after ischemic stroke: the effect of acute and post-acute care-pathways in a cohort study. BMC Public Health 2016;16:408. [Crossref] [PubMed]
- Gabet A, Grimaud O, de Peretti C, et al. Determinants of Case Fatality After Hospitalization for Stroke in France 2010 to 2015. Stroke 2019;50:305-12. [Crossref] [PubMed]
- Read SH, McAllister DA, Colhoun HM, et al. Incident ischaemic stroke and Type 2 diabetes: trends in incidence and case fatality in Scotland 2004-2013. Diabet Med 2018;35:99-106. [Crossref] [PubMed]
- Sennfält S, Norrving B, Petersson J, et al. Long-Term Survival and Function After Stroke. Stroke 2018; Epub ahead of print. [Crossref] [PubMed]
- OECD iLibary. Health at a Glance 2019: OECD Indicators. Mortality following ischaemic stroke. Available online: https://www.oecd-ilibrary.org/sites/a489af86-en/index.html?itemId=/content/component/a489af86-en#:~:text=Across%20OECD%20countries%2C%207.7%25%20of,with%20mortality%20rates%20over%2012%25. Accessed May 25, 2022.
- Zhang R, Wang Y, Fang J, et al. Worldwide 1-month case fatality of ischaemic stroke and the temporal trend. Stroke Vasc Neurol 2020;5:353-60. [Crossref] [PubMed]
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167-76. [Crossref] [PubMed]
- Zahuranec DB, Lisabeth LD, Sánchez BN, et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology 2014;82:2180-6. [Crossref] [PubMed]
- Béjot Y, Grelat M, Delpont B, et al. Temporal trends in early case-fatality rates in patients with intracerebral hemorrhage. Neurology 2017;88:985-90. [Crossref] [PubMed]
- Huang H, Lai LT. Incidence and Case-Fatality of Aneurysmal Subarachnoid Hemorrhage in Australia, 2008-2018. World Neurosurg 2020;144:e438-46. [Crossref] [PubMed]
- Rehman S, Phan HT, Reeves MJ, et al. Case-Fatality and Functional Outcome after Subarachnoid Hemorrhage (SAH) in INternational STRoke oUtComes sTudy (INSTRUCT). J Stroke Cerebrovasc Dis 2022;31:106201. [Crossref] [PubMed]
- Cross SH, Kaufman BG, Warraich HJ. Trends in Location of Death for Individuals With Cerebrovascular Disease in the United States. JAMA Neurol 2019;76:1399-401. [Crossref] [PubMed]
- Dasch B, Lenz P. The Place of Death of Neurological Patients with Selected Disease Entities: Data from an Observational Study on Places of Death from Germany. Fortschr Neurol Psychiatr 2022;90:447-55. [PubMed]
- Ward A, Payne KA, Caro JJ, et al. Care needs and economic consequences after acute ischemic stroke: the Erlangen Stroke Project. Eur J Neurol 2005;12:264-7. [Crossref] [PubMed]
- Schneider K, Heise M, Heuschmann P, et al. Lebens- und Versorgungssituation von Schlaganfallpatienten. Nervenheilkunde 2009;28:114-8. [Crossref]
- Ullberg T, Zia E, Petersson J, et al. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke 2015;46:389-94. [Crossref] [PubMed]
- Federal Statistical Office of Germany (Destatis) (2021). Hospital patients - Germany, years, main diagnoses ICD-10. Available online: https://www-genesis.destatis.de/genesis//online?operation=table&code=23131-0001&bypass=true&levelindex=0&levelid=1636899464777#abreadcrumb. Accessed May 25, 2022.
- Heuschmann PU, Busse O, Wagner M, et al. Schlaganfallhäufigkeit und Versorgung von Schlaganfallpatienten in Deutschland. Akt Neurol 2010;37:333-40. [Crossref]
- Krogias C, Bartig D, Kitzrow M, et al. Trends of hospitalized acute stroke care in Germany from clinical trials to bedside. Comparison of nation-wide administrative data 2008-2012. J Neurol Sci 2014;345:202-8. [Crossref] [PubMed]
- Misselwitz B, Grau A, Berger K, et al. Versorgungsqualität des akuten ischämischen Schlaganfalls in Deutschland. Nervenarzt 2020;91:484-92. [Crossref] [PubMed]
- Stevens T, Payne SA, Burton C, et al. Palliative care in stroke: a critical review of the literature. Palliat Med 2007;21:323-31. [Crossref] [PubMed]
- Le BH, Pisasale M, Watt J. Palliative care in stroke. Palliat Med 2008;22:95-6. [Crossref] [PubMed]
- Burton CR, Payne S, Addington-Hall J, et al. The palliative care needs of acute stroke patients: a prospective study of hospital admissions. Age Ageing 2010;39:554-9. [Crossref] [PubMed]
- Gerhard C. Kapitel 5. Typische neurologische Krankheitsbilder mit palliativem Versorgungsbedarf - Schlaganfall. In: Chr. Gerhard (Hrsg.). Neuro-Palliative Care. Bern: Verlag Hans Huber, 2011:303-17.
- Creutzfeldt CJ, Holloway RG, Walker M. Symptomatic and palliative care for stroke survivors. J Gen Intern Med 2012;27:853-60. [Crossref] [PubMed]
- Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1887-916. [Crossref] [PubMed]
- Creutzfeldt CJ, Holloway RG, Curtis JR. Palliative Care: A Core Competency for Stroke Neurologists. Stroke 2015;46:2714-9. [Crossref] [PubMed]
- Braun LT, Grady KL, Kutner JS, et al. Palliative Care and Cardiovascular Disease and Stroke: A Policy Statement From the American Heart Association/American Stroke Association. Circulation 2016;134:e198-225. [Crossref] [PubMed]
- Singh T, Peters SR, Tirschwell DL, et al. Palliative Care for Hospitalized Patients With Stroke: Results From the 2010 to 2012 National Inpatient Sample. Stroke 2017;48:2534-40. [Crossref] [PubMed]
- Molidor S, Overbaugh KJ, James D, et al. Palliative Care and Stroke: An Integrative Review of the Literature. J Hosp Palliat Nurs 2018;20:358-67. [Crossref] [PubMed]
- Steigleder T, Kollmar R, Ostgathe C. Palliative Care for Stroke Patients and Their Families: Barriers for Implementation. Front Neurol 2019;10:164. [Crossref] [PubMed]
- Cowey E, Schichtel M, Cheyne JD, et al. Palliative care after stroke: A review. Int J Stroke 2021;16:632-9. [Crossref] [PubMed]
- Gao L, Zhao CW, Hwang DY. End-of-Life Care Decision-Making in Stroke. Front Neurol 2021;12:702833. [Crossref] [PubMed]
- Comer AR, Williams LS, Bartlett S, et al. Palliative and End-of-Life Care After Severe Stroke. J Pain Symptom Manage 2022;63:721-8. [Crossref] [PubMed]
- Chauhan N, Ali SF, Hannawi Y, et al. Utilization of Hospice Care in Patients With Acute Ischemic Stroke. Am J Hosp Palliat Care 2019;36:28-32. [Crossref] [PubMed]
- duPreez AE, Smith MA, Liou JI, et al. Predictors of hospice utilization among acute stroke patients who died within thirty days. J Palliat Med 2008;11:1249-57. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Place of death after stroke--United States, 1999-2002. MMWR Morb Mortal Wkly Rep 2006;55:529-32. [PubMed]
- Institute for Epidemiology and Social Medicine, University of Münster, Germany. Quality Assurance Stroke Northwest Germany. Annual evaluation 2020 [Qualitätssicherung Schlaganfall Nordwestdeutschland. Jahresauswertung 2020][German]. Available online: https://www.medizin.uni-muenster.de/qsnwd/downloads.html. Accessed May 25, 2022.
- Radbruch L, Payne S. White Paper on Standards and Norms for Hospice and Palliative Care in Europe: Part 2. Recommendation of the European Association for Palliative Care. Z Palliativmed 2011;12:260-70. [Crossref]
- National Hospice and Palliative Care Organization (NHPCO) (2020). NHPCO Facts and Figures 2020 Edition. file:///C:/Users/burkh/A_Sonstige_Ordner/Downloads/NHPCO-Facts-Figures-2020-edition.pdf. Accessed May 25, 2022.
- Melching, H. Palliative care for non-tumor patients - ambition and reality. [Palliativversorgung für Nichttumorpatienten – Anspruch und Wirklichkeit][German]. Available online: https://www.google.com/search?rlz=1C1CHBF_deDE884DE884&sxsrf=APq-WBsdLszMqbUDjSC7vDob9pa6fEjIWg:1645911119220&q=palliativversorgung+f%C3%BCr+Nicht+Tumorpatienten&spell=1&sa=X&ved=2ahUKEwjZ2t7cqJ72AhVkSfEDHZwVANkQBSgAegQIARA3&biw=1366&bih=625&dpr=1. Accessed May 25, 2022.
- Bar B, Creutzfeldt CJ, Rubin MA. Palliative Care in the Neuro-ICU: Perceptions, Practice Patterns, and Preferences of Neurointensivists. Neurocrit Care 2020;32:302-5. [Crossref] [PubMed]
- Muehlschlegel S. When Doctors and Families Disagree in the Neurologic Intensive Care Unit-Misunderstandings and Optimistic Beliefs. JAMA Netw Open 2021;4:e2129079. [Crossref] [PubMed]
- Ostgathe C, Alt-Epping B, Golla H, et al. Non-cancer patients in specialized palliative care in Germany: what are the problems? Palliat Med 2011;25:148-52. [Crossref] [PubMed]
- Hess S, Stiel S, Hofmann S, et al. Trends in specialized palliative care for non-cancer patients in Germany--data from the national hospice and palliative care evaluation (HOPE). Eur J Intern Med 2014;25:187-92. [Crossref] [PubMed]
- Holloway RG, Ladwig S, Robb J, et al. Palliative care consultations in hospitalized stroke patients. J Palliat Med 2010;13:407-12. [Crossref] [PubMed]
- Tran LN, Back AL, Creutzfeldt CJ. Palliative Care Consultations in the Neuro-ICU: A Qualitative Study. Neurocrit Care 2016;25:266-72. [Crossref] [PubMed]
- Williams MT, Zimmerman E, Barry M, et al. A Retrospective Review of Patients With Acute Stroke With and Without Palliative Care Consultations. Am J Hosp Palliat Care 2019;36:60-4. [Crossref] [PubMed]
- Federal Ministry of Justice of Germany (2015). Act to improve hospice and palliative care. [Gesetz zur Verbesserung der Hospiz- und Palliativversorgung. Hospiz- und Palliativgesetz (HPG)][German]. Available online: https://www.ilo.org/dyn/natlex/natlex4.detail?p_lang=en&p_isn=101621. Accessed May 25, 2022.
- Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;CD007760. [PubMed]
- Bajwah S, Oluyase AO, Yi D, et al. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2020;9:CD012780. [PubMed]
- Yadav S, Heller IW, Schaefer N, et al. The health care cost of palliative care for cancer patients: a systematic review. Support Care Cancer 2020;28:4561-73. [Crossref] [PubMed]



