
Early pregnancy combined with acute hematogenous disseminated pulmonary tuberculosis leading to stillbirth: a case report
Introduction
Pregnancy with tuberculosis (PWT) refers to a group of conditions caused by Mycobacterium tuberculosis (TB) infection during pregnancy or by pregnancy with uncured TB. The incidence of PWT is approximately 5–7% (1,2). In China, with the liberalization of the two-child policy and the increase of in vitro fertilization (IVF), PWT cases have increased significantly, but the level of treatment has stagnated in the 1980s–1990s. It is usually difficult to diagnose PWT given the non-specificity of the symptoms and the complex situation surrounding pregnancy. The delayed diagnosis of PWT is common in clinical practice (1,2). 2HREZ/4HR scheme (isoniazid, rifampicin, pyrazinamide and ethambutol for 2 months, isoniazid and rifampicin for 4 months) is often used to treat PWT. This scheme has been proved to be very safe for pregnant women during pregnancy and is the first choice for newly diagnosed patients with TB.
PWT affects the normal growth and development of the mother and fetus, leading to premature births, low-birth-weight infants, and perinatal deaths (1,2). The timely diagnosis of PWT and sufficient anti-TB treatment are crucial to ensuring good pregnancy outcomes in patients. Antenatal care and nutritional therapy are also helpful in achieving this goal (1,2). We reported a case of early pregnancy with hematogenous disseminated pulmonary TB leading to fetal death. This report seeks to raise clinicians’ awareness about the diagnosis and management of this complex condition, thereby ensuring maternal and fetal safety. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-798/rc).
Case presentation
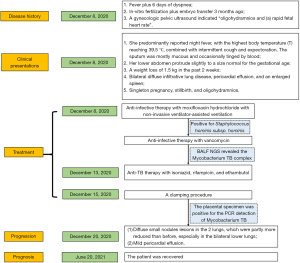
The 35-year-old G2P1 pregnant woman at 14 weeks of gestation was admitted to the hospital on December 8, 2020, following 2 weeks of fever plus 6 days of dyspnea. Some 2 years ago, she achieved a satisfactory postpartum recovery following laparoscopic tubal recanalization for blocked fallopian tubes. The patient underwent IVF plus embryo transfer 3 months ago. There was 1 report of mild vaginal bleeding. A gynecologic pelvic ultrasound indicated “oligohydramnios and (a) rapid fetal heart rate”, which were left untreated.
When the patient presented, she complained of having suffered from a fever of unknown origin for more than 2 weeks. She predominantly reported night fever, with the highest body temperature (T) reaching 39.5 ℃, combined with intermittent cough and expectoration. The sputum was mostly mucous and occasionally tinged by blood. The patient sought healthcare at another hospital, and the cough symptoms improved after cefuroxime treatment. However, an intermittent fever persisted. She had another episode of high fever, reaching 38.9 ℃, 6 days before her admission. She complained of chest tightness and shortness of breath after physical exertion, which remained unmitigated after rest.
A physical examination on admission revealed the following: T 38.4 ℃, respiratory rate 44 breaths/min, pulse 134 beats/min, blood pressure 119/74 mmHg, and SPO2 92%. The breathing sounds were slightly harsh in the 2 lungs. No dry or wet rales were heard. Her lower abdomen protruded slightly to a size normal for the gestational age. She had mild edema in the lower extremities. She reported a weight loss of 1.5 kg in the past 2 weeks and denied a history of TB or typhoid.
Routine blood tests on admission found the following: white blood cell count 10.85×109/L, neutrophil percentage 89.5%, lymphocyte percentage 5.2%, D-dimer level 5.82 µg/mL, procalcitonin 0.549 ng/mL, interleukin (IL)-6 67.5 pg/mL, and C-reactive protein 88.74 mg/L. The cluster of differentiation (CD) marker panel revealed a CD3+ cell count of 264 cells/µL, a CD8+ cell count of 106 cells/µL, a CD4/CD8 ratio of 1.38, and a CD4+ cell count of 146 cells/µL. The results of the tuberculin skin test were negative. No apparent abnormalities were found by the erythrocyte sedimentation rate test, antinuclear antibody profile, antineutrophil cytoplasmic antibody profile, anti-CCP antibody test, rheumatoid factor test, G-test, GM test, cytomegalovirus DNA test, and EB virus DNA test. Based on these results, the possibility of a viral infection or autoimmune disease was excluded. The blood gas analysis showed FiO2 41%, PH 7.48, PO2 65 mmHg, PCO2 30 mmHg, and HCO3− 25 mmol/L.
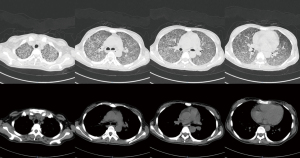
The chest computed topography (CT) scan showed (I) bilateral diffuse infiltrative lung disease, (II) pericardial effusion, and (III) an enlarged spleen (see Figure 1). No signs of pulmonary embolism were found by pulmonary CT angiography. Cardiac ultrasonography indicated (I) mild pericardial effusion, and (II) mild tricuspid regurgitation. An abdominal B-mode ultrasound and head CT did not reveal any apparent abnormalities. A pelvic CT scan revealed intrauterine pregnancy and mild exudation from the abdominal or pelvic cavities. An abdominal gynecological ultrasound indicated (I) singleton pregnancy, stillbirth, and oligohydramnios; (II) no abnormalities in the appendices. The patient was preliminarily diagnosed with severe pneumonia, TB, and inevitable miscarriage.
On admission, she received anti-infective therapy with moxifloxacin hydrochloride with non-invasive ventilator-assisted ventilation. Additionally, 2 blood cultures were positive for Staphylococcus hominis subsp. hominis (methicillin-resistant coagulase-negative Staphylococci). Staphylococcal bloodstream infection was considered, and the patient received anti-infective therapy with vancomycin. The patient’s oxygenation improved, but her body temperature fluctuated between 38.2 ℃ and 38.5 ℃. Bronchoalveolar lavage fluid (BALF) was negative for PCR detection of Mycobacterium TB. BALF next-generation sequencing (NGS) revealed the Mycobacterium TB complex (sequence No. 6).
Anti-TB therapy with isoniazid, rifampicin, and ethambutol started on December 13, 2020. A clamping procedure was performed on December 15, 2020. The placental specimen was positive for the PCR detection of Mycobacterium TB (see Figure 2). A chest CT re-examination on December 20, 2020, revealed (I) diffuse small nodules lesions in the 2 lungs, which were partly more reduced than before, especially in the bilateral lower lungs, and (II) mild pericardial effusion (see Figure 3). The diagnosis of PWT was confirmed based on other examinations. The patient subsequently received regular anti-TB therapy. After the discharge, the patient received outpatient follow-up for 6 months and she was recovered. The timeline axis was shown in Figure 4.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PWT refers to a group of conditions caused by Mycobacterium TB infection during pregnancy or by pregnancy in patients with uncured TB. The population affected by TB has been growing rapidly due to the rise of the acquired immunodeficiency syndrome (AIDS) epidemic and the increasing prevalence of multidrug-resistant Mycobacterium TB (3-5). The incidence of TB is higher in developing countries than developed countries due to economic and medical backwardness (1-7). A large-scale retrospective study showed that among females from countries in which TB is prevalent, the incidence of TB is 0.137% in unpregnant and non-postpartum women, 0.182% in pregnant women, and 0.233% in postpartum women. Thus, TB represents a major health threat during pregnancy. PWT is a likely cause of adverse pregnancy outcomes, such as preterm births and stillbirths (1,2).
It has been reported that the delayed diagnosis of PWT is common given the non-specificity of symptoms and the complex situation of pregnancy (1,2). This is especially true in asymptomatic pregnant women with TB (8). Mycobacterium TB can spread to the endometrium via the bloodstream in pregnant women with acute hematogenous disseminated pulmonary TB. This condition can cause severe damage to the fetus, resulting in fetal ischemia and hypoxia, developmental delays, premature labor, and even abortion (1,2,9). Thus, early diagnosis and timely treatment are essential to improve the prognosis of pregnant women and decrease fetal mortality.
WHO defines TB screening as “systematic identification of suspected active tuberculosis patients”. that is, in the pre-determined target group, screening should be carried out through the examination or other procedures that can be quickly applied. For women of childbearing age who have childbearing requirements, such as having high-risk factors of TB infection, they should assess TB by assessing symptoms for physical examination and determining TB risk factors before pregnancy. The common TB screening tools recommended by who are four symptom screening tools (cough, fever, night sweat and weight loss). Any one of these four symptoms is identified as screening positive. The presenting symptoms of our reported patient included intermittent fever and dyspnea. Fever and weight loss were already conspicuous at the gestational age of 14 weeks. However, the severity of the condition was underestimated. No further examinations or treatments were given except for cefuroxime, resulting in delayed diagnosis and treatment. The disease progressed, and the fetus died. Clinicians should be particularly aware of fever and dyspnea during pregnancy and confirm the diagnosis as soon as possible.
A variety of tools are available to improve the diagnostic accuracy of PWT, including the tuberculin skin test, bacteriological examination of sputum, molecular Xpert MTB/RIF test, as well as chest X-ray, CT, magnetic resonance imaging, and radiographic methods (10-13). Other test methods, such as interferon-γ release assay, PCR for the detection of Mycobacterium TB, and the NGS of BALF, can also assist with the diagnosis (10). Our reported case, affected by hypo-immunity, had false negative results for the tuberculin skin test. However, she tested positive for the PCR detection of Mycobacterium TB in the pathological placental tissues. Additionally, the Mycobacterium TB complex was present in the BALF according to the NGS. Diffuse nodular shadows were found by radiographic examinations bilaterally. The combined use of several diagnostic tools provides an important basis for the confirmation of PWT.
The early diagnosis and anti-TB treatment of PWT are very important for PWT. Studies have shown that pregnancy outcomes improve if PWT is diagnosed and treated earlier (1,2,8,9). A sufficient prenatal examination is conducive to early diagnosis (8). Anti-TB treatment before delivery can effectively delay TB progression, reducing the possibility of it developing into severe TB and newborn morbidity. Anti-TB treatment before delivery is superior to postpartum therapy (14).
As for the treatment for those with drug-sensitive PWT, the World Health Organization recommends the standard 2HRZE/4HR regimen during pregnancy (15,16). Quinolones, protionamide, and bedaquiline can be used for multi-drug-resistant PWT (17-19). The nutritional therapy delivered along with the anti-TB treatment can help improve the health status of the mother and the infant (1,2).
In the present case, intrauterine death had already occurred before the anti-TB treatment commenced. Fortunately, the lesions in the 2 lungs were effectively controlled with the combined use of isoniazid, rifampicin, and ethambutol once the diagnosis was confirmed. The patient was discharged from the hospital after her condition improved. As discussed above, early and effective anti-TB treatment can delay disease progression and improve a patient’s prognosis.
To conclude, the early diagnosis and treatment of PWT are critical for both the mother and the fetus. Clinicians should have comprehensive knowledge of PWT. The combined use of several examination tools at an early stage is needed to diagnose PWT. The active treatment and management of PWT, including multidrug-resistant TB, will contribute to maternal and fetal health.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-798/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-798/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Orazulike N, Sharma JB, Sharma S, et al. Tuberculosis (TB) in pregnancy - A review. Eur J Obstet Gynecol Reprod Biol 2021;259:167-77. [Crossref] [PubMed]
- Jana N, Barik S, Arora N, et al. Tuberculosis in pregnancy: the challenges for South Asian countries. J Obstet Gynaecol Res 2012;38:1125-36. [Crossref] [PubMed]
- Bates M, Ahmed Y, Kapata N, et al. Perspectives on tuberculosis in pregnancy. Int J Infect Dis 2015;32:124-7. [Crossref] [PubMed]
- Seung KJ, Keshavjee S, Rich ML. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb Perspect Med 2015;5:a017863. [Crossref] [PubMed]
- Mokhele I, Jinga N, Berhanu R, et al. Treatment and pregnancy outcomes of pregnant women exposed to second-line anti-tuberculosis drugs in South Africa. BMC Pregnancy Childbirth 2021;21:453. [Crossref] [PubMed]
- World Health Organization. Global tuberculosis report 2016. Geneva, Switzerland: World Health Organization, 2016.
- El-Messidi A, Czuzoj-Shulman N, Spence AR, et al. Medical and obstetric outcomes among pregnant women with tuberculosis: a population-based study of 7.8 million births. Am J Obstet Gynecol 2016;215:797.e1-6. [Crossref] [PubMed]
- Duan C, Yu YL. Research progress of pregnancy combined with tuberculosis. Chinese Journal of Infectious Diseases 2018;36:317-20.
- Han D, Chen ZF, Liu YL, et al. The effects of anti-TB treatment on the growth and development of infants born by maternal active TB cases during terminal stage of pregnancy. The Journal of The Chinese Antituberculosis Association 2013;35:361-4.
- Nhan-Chang CL, Jones TB. Tuberculosis in pregnancy. Clin Obstet Gynecol 2010;53:311-21. [Crossref] [PubMed]
- Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 2015;3:451-61. [Crossref] [PubMed]
- Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2017;130:e210-6. [Crossref] [PubMed]
- Schloß M, Heckrodt J, Schneider C, et al. Magnetic Resonance Imaging of the Lung as an Alternative for a Pregnant Woman with Pulmonary Tuberculosis. J Radiol Case Rep 2015;9:7-13. [Crossref] [PubMed]
- Sun XL, Ren BY, Xu JL. Effects of treatment timing on the outcome of pregnant women combined with pulmonary tuberculosis. Chinese Journal of Antituberculosis 2016;38:747-50.
- Health Protection Agency. Pregnancy and Tuberculosis: Guidance for Clinicians. London, UK: Health Protection Agency; 2006.
- Laniado-Laborín R, Carrera-López K, Hernández-Pérez A. Unexpected Pregnancy during Treatment of Multidrug-resistant Tuberculosis. Turk Thorac J 2018;19:226-7. [Crossref] [PubMed]
- Hu HY, Dong RF, Xu FS. Diagnosis and treatment of late pregnancy combined with active tuberculosis. Chinese Journal of Woman and Child Health Research 2017;28:733-5.
- Loveday M, Hughes J, Sunkari B, et al. Maternal and Infant Outcomes Among Pregnant Women Treated for Multidrug/Rifampicin-Resistant Tuberculosis in South Africa. Clin Infect Dis 2021;72:1158-68. [Crossref] [PubMed]
- Garcia-Prats AJ, Draper HR, Finlayson H, et al. Clinical and Cardiac Safety of Long-term Levofloxacin in Children Treated for Multidrug-resistant Tuberculosis. Clin Infect Dis 2018;67:1777-80. [Crossref] [PubMed]




