Pathophysiology of nausea and vomiting in palliative medicine
Abstract: Nausea/vomiting remains a significant problem in medicine, especially in patients with chronic
illnesses. The incidence and patient distress level from nausea and vomiting are underestimated by health
care providers. A thorough patient evaluation followed by rational polypharmacy and a multimodal treatment
approach may minimize the occurrence and intensity of nausea/vomiting.
Utilizing new techniques (e.g., PET imaging of the CNS with novel radiotracers, functional Magnetic
Resonance Imaging), clinicians could have a greater chance to elucidate which receptors may be contributing
to an individual’s experience of nausea and vomiting and the relative importance of each.
Future research into assessing precise mechanisms of nausea and vomiting in particular patients may
enable clinicians to design the most appropriate combinations of antiemetics in efforts to achieve the most
effective therapy with the least side effects. Individually-tailored antiemetic “cocktails,” based on patient
specific pathophysiology, may lead to optimal treatment outcomes.
Key words: Nausea; vomiting; NK-1 antagonists; 5HT-3 antagonists
Nausea has been considered a uniquely unpleasant discomfort that defies precise definition (1). Although this statement may be reasonably accurate, it has little clinical utility.
Retching consists of spasmodic inspiratory movements with the glottis closed and abdominal muscle contractions such that the pressure generated by the abdominal musculature is opposed by negative intrathoracic pressure (the gastric antrum contracts - while fundus and cardiac relax). Vomiting is the forceful expulsion of the gastric contents out of the mouth from a coordinated contraction of predominantly abdominal muscles and diaphragm while the gastric cardia is open and elevated with contracted pylorous (2).
Smith has defined nausea as an unpleasant sensory and emotional experience, which may be described in terms of a “sick” feeling with or without a sense of impending vomiting/ retching - often associated with a perception of epigastric or upper abdominal unpleasantness or awareness (3). Nausea may be accompanied by autonomic-driven physiologic changes of pallor, diaphoresis, altered heart rate (tachycardia or bradycardia), upper GI tract hypersecretion, and relaxation of the gastric fundus and cardia (2). Although it is common for nausea to be followed by a retching/vomiting phase, vomiting can occur without any preceding nausea and nausea can come and go without any retching/vomiting (2). Nausea is a common symptom experienced in many diseases and their treatment can be quite severe and disabling; however, it is sometimes given minimal attention by healthcare providers who may view nausea as an annoying side effect and not as a significant patient problem or major issue to address/treat. The onset of nausea/vomiting may be acute or gradual and the perception of nausea may be constant or intermittent. If nausea/vomiting is constant, it may be steady or wax and wane. Severe persistent nausea/ vomiting may lead to significant adverse effects including: dehydration, electrolyte imbalance, malnutrition, and significant deterioration in quality of life (QOL).
It is important to document the perceived level of nausea intensity. Most commonly in clinical practice this is done with unidimensional tools that are also used for pain evaluation which include: category scales with “verbal” descriptors (e.g., mild, discomforting, distressing, horrible, excruciating) (4) or (mild, moderate, severe); numeric rating scales (NRS-11) - a simple commonly used scale in which patients indicate how intense their nausea is by selecting a number from one to ten (where 0 represents “no nausea at all,” and 10 represents “the worst nausea imaginable”); and visual analog scales (VAS - in which the patient marks a 10-cm line anchored at one end by “no nausea” and at the other end by “worst nausea imaginable”). A more elaborate instrument which is multidimensional and valid if subscales are formed to reflect the multidimensional structure of nausea and vomiting in pregnancy is the Rhodes Index of Nausea and Vomiting-Form 2 (Rhodes INV2) (5); however, this is specific for pregnancy.
The Functional Living Index-Emesis (FLIE) is another instrument to assess the functional impact of emesis and its treatment on the lives of patients. Martin and colleagues utilizing the FLIE tool demonstrated that improved control of emesis (with the NK1 receptor antagonistaprepitant) was highly effective in reducing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives (6).
The level of distress/suffering from nausea/vomiting perceived by the patient varies dramatically and should not be underestimated. Certain patients are so bothered by nausea that when given a choice they would rather have no nausea and tolerate some degree of pain. Grunberg and colleagues have demonstrated the impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score (7). Other investigators have also shown significant effects on quality of life. Terauchi et al. (8) suggested that: utilizing orally disintegrating antiemetic tablets (ramosetron) in efforts to diminish chemotherapy-induced delayed nausea and vomiting in patients with recurrent gynecologic malignancies was useful for improving quality of life (Ishihara’s QOL survey method) (9).
The degree/intensity of symptoms as well as the distress/suffering which a symptom causes (which may be potentially modulated by patient-specific adaptive coping mechanisms and patient-specific goal-directed motivation/ drive) contributes to the impact of that symptom on the patient’s life and therefore both should be documented. The symptom-related impact on life (SRIL) (including impact on the patient’s functioning) may greatly affect the patient’s quality of life. These effects on quality of life may be very different depending on the individual patient. Additionally, pre-existing disability/impairments (if any), as well as the degree of change in disability/impairment (if any), may impact QOL.
Cleeland et al. have described the quantitative assessment of pain-related distress using a numeric rating scale-11 (NRS-11) (with zero being no distress and ten being the worst distress imaginable) (10). Although the level of pain intensity affects pain-related distress, painrelated distress may in turn have a significant impact on pain intensity, duration, and secondary outcome (10). In the case of nausea, no such nausea-specific distress rating is routinely used in clinical practice, although it is important to assess. Nausea-related distress should be documented utilizing an NRS-11 scale (with zero being no distress and ten being the worst distress imaginable). Nausearelated distress represents how bothersome the nausea experienced is to the individual patient or the amount of anguish caused by nausea.
Receptors that may modulate nausea/vomiting
Borison and Wang postulated the existence of a discrete vomiting center located in the medulla (11,12); however, Miller and Wilson (13) [via stimulation of the nucleus tractus solitarius or nucleus of the solitary tract (NTS) and reticular formation] could not identify any discrete locus and concluded that the neural circuitry involved in emetic responses is diffusely distributed in and around the region described by Borison and Wang (11,12).
There is no well-defined discrete vomiting center. The terminology “vomiting center” should be replaced with the term “emetic complex” (EC) - to refer to groups of loosely organized neurons distributed throughout the medulla which are sequentially activated by a central pattern generator (CPG) and play a role in emesis.
The EC is composed of the prodromal-sign center (PSC) (located in the reticular area dorsally adjacent to the semi compact part of the nucleus ambiguous) and the central pattern generator center (CPGC) [located dorsomedial to the retrofacial nucleus (RFN)]. The PSC predominantly consists of CPG-driving neurons and prodromal-sign neurons. The CPGC (for vomiting) appears to have afferent areas driving expulsion and retching (2).
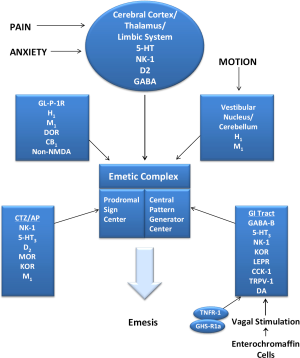
The emetic reflex (ER) is considered a defense mechanism (with significant autonomic nervous system involvement) in order to rid toxins/noxious agents from the gastrointestinal (GI) system prior to absorption (2). Rather than a vomiting center located in a specific location, the emetic reflex arc consists of essentially five major parts which contribute to and/or coordinate the ER and are distributed through the medullary and brainstem areas. The five major parts of the ER arc are: the vestibular nuclei and cerebellum (VN/C); the higher central nervous system (CNS) centers [including cerebral cortex and limbic system (CC/LS)]; the nucleus of the solitary tract or nucleus tractus solitarius (NTS); the chemoreceptor trigger zone/area postrema (CTZ/AP); and the emetic complex (EC). The VN/C, CC/LS, NTS, and CTZ/AP are thought to all eventually “feed into” the final common pathway - the emetic complex (Figure 1).
There are three major lines of defense that humans have against toxin or noxious agent gaining enteral access to the internal milieu of the body. The first line of defense is aimed at preventing the ingestion of toxins/noxious agents into the GI system and entails sight, task, smell, hearing, anxiety/memory, and vestibular labyrinth mostly from VN/ C and CC/LS parts (2). The second line of defense is aimed at preventing the absorption of toxins/noxious agents and entails the NTS which is the sensory nucleus of the vagus nerve and glossopharyngeus nerve. The vagus nerve receives afferent signals from almost all parts of the upper digestive organs and is located posterior to the emetic complex (2).
The third line of defense is aimed at sensing toxins/ noxious agents in the circulation and entails the CTZ of the area postrema (CTZ/AP). The CTZ/AP located on the floor of the fourth ventricle has a dual detection function. Chemoreceptors facing the ventricle are directly exposed to toxins/noxious agents in the cerebrospinal fluid (CSF) (2). Also, there exists a dense vascular network of fenestrated capillaries which allow detection of circulating irritants which would not pass through the blood- brain barrier (2). Chemoreceptors are additionally present in the area postrema which are outside the blood brain barrier and sensitive to toxins/noxious agents.
Vagal afferent fibers possess a variety of receptors which can facilitate (e.g., 5-HT3, CCK1, TRPV1, NK1) or diminish (e.g., ghrelin, leptin, KOR, GABA-B) neural activity (14). A complex intricate network of signals affect human appetite/satiety/food intake. It is conceivable that certain peptides/hormones that affect appetite may contribute to the perception of nausea in some circumstances. Many of these peptides/hormones are released from the gut {e.g., oxyntomodulin and GLP-1 [which both bind to the GLP-1 receptor (GLP- 1R)], peptide YY, ghrelin (which binds to the GHSR) particularly in the postprandial period} (15).
Ghrelin, a gastric peptide, which possesses orexigenic effects, is the endogenous ligand for the growth hormone secretagogue receptor (GHSR) with stimulating effects on growth hormone and gastrointestinal motility (16). Gaskin and colleagues demonstrated that a sub-threshold dose (12.5 mg/kg; SC) of N(omega)-nitro-L-arginine methyl ester (L-NAME) [a nitric oxide snythase (NOS) inhibitor] significantly blocked the ghrelin-induced increase in food intake. The administration of ghrelin increased NOS levels in the hypothalamus-supporting the hypothesis that ghrelin’s effects are nitric oxide dependent (16).
Hermann and colleagues hypothesized that tumor necrosis factor alpha (TNFα), acting on the neural circuitry of the medullary dorsal vagal complex (DVC), may lead to altered gastric function with possible gastric stasis, anorexia, nausea, and vomiting (17). Microinjections of TNFR:Fc (TNFR:Fc; TNF-receptor linked to the Fc portion of the human immunoglobulin IgG1 - which neutralizes the suppressive effects of endogenous TNF-alpha), an adsorbent construct in the central nervous system, suppressed induction of NTS cFos immunoreactivity normally evoked by TNFα (17). The transmission of emetic signals between visceral vagal afferent neurons and the second-order neurons of the NTS may be mediated by glutamate binding to non-N-methy1-D-aspartate (NMDA) receptors in dogs (18).
The caudal nucleus of the NTS processes preproglucagon to glucagons-like peptides (GLP)-1 and-2 which inhibit food intake when given intracerebroventricularly (19). GLP-1/2-containing neuronal circuitry seems to constimulates these neurons, and LiCl-induced suppression of food intake is blocked by the GLP-1 receptor antagonist exendin-9 (19). Vrang et al. demonstrated that gastric distention (via balloon in non anesthetized freely moving rats) produced significant increases in c-Fos-expressing NTS neurons (19). Fundus and corpus distention increased the percentage of c-Fos-activated GLP-1 neurons to 21±9% and 32±5% compared with 1±1% with sham distention (P<0.01) (19).
The precise role of the neurokinin 1 (NK) receptor and NK1 receptor antagonists in emesis and its treatment remains uncertain. HSP-117, an NK1 receptor antagonist with antiemetic activity, inhibited the substance P-induced discharge of action potentials of single NTS neuron recorded in slices of ferret brainstem (20), suggesting that the site of action of NK1 receptor antagonists may be the NTS. However, this site is more likely where NTS second-order neurons activate the prodomal-sign center for vomiting (located in the reticular area dorsally adjacent to the semi compact part of the nucleus ambiguous) via NK1 receptors (21). Although the major site of action for the effects of many antiemetics appears to be central, it is conceivable that peripheral actions may contribute to antiemetic effects as well. Gastric dopamine (D2) receptors are involved in inhibiting gastric motility during nausea/ vomiting and represent a potential peripheral target for dopamine (D2) receptor antagonists (2).
5-HT3 receptor antagonists, although having a major action on the CTZ, also may dampen the ER afferent input and transmission by inhibiting presynaptic vagal 5-HT3 receptors, blocking 5-HT enterochromaffin cell autoreceptors (thereby inhibiting 5-HT release), and impeding transmission of emetic afferent input in vagus nerve nuclei (22-24).
Additionally, although the anti-emetic actions of NK1 receptor antagonists appear to be largely (if not entirely) central–it is theoretically conceivable that the inhibition of NK1 receptors of vagal motor neurons [which inhibit fundic relaxation (a prodomal event before vomiting)] may contribute as well (21).
Figure 1 illustrates some of the major features of the ER in an attempt to give clinicians a rudimentary “roadmap” of the ER and a “blueprint” for where various anti-emetics may act. This simplistic depiction of the ER illustrates the major parts involved in the ER arc but the actual neural network/circuitry which contributes to the ER arc is extremely complex and there are other areas/parts of the CNS involved as well as crosstalk among the 5 major parts of the ER. There are also multiple receptors which are not depicted and many receptors which are located in multiple areas which are not shown in Figure 1.
Additionally, afferent signals (e.g., from NTS, CTZ/AP) are connected to pre-motor (nucleus retroambiguous) and motor (dorsal vagus, phrenic nuclei) efferent signals which lead to the act of vomiting (2).
Potential receptor interactions in nausea/vomting
In 1991, ondansetron became available revolutionizing the prevention of acute emesis. Other 5-HT3 receptor antagonists like granisetron and dolasetron soon followed; even though they exhibited differences in 5-HT3 receptor binding affinity, serum halflife, and metabolism, they exhibited similar control on acute emesis compared to ondansetron and had no major effect on delayed emesis (25). These clinical results led to the hypothesis that serotonin plays a central role in the mechanism of acute emesis but a lesser role in the pathogenesis of delayed emesis (26).
Aprepitant introduced in 2003, counteracts the activity of SP, the preferred ligand at NK1 receptors. These receptors are located in the gut, the area postrema and the nucleus tractus solitarius; all areas involved in the emetic reflex. Like serotonin, SP is released by emetogenic chemotherapies but it appears to act largely on receptors that are centrally located. Consequently, NK1 receptor antagonists require entry into the central nervous system to have an antiemetic effect (26,27).
Unlike other 5-HT3 receptor antagonists, palonosetron in addition to competing with serotonin exhibits allosteric binding and positive cooperativity (28). Allosteric binding induces a conformational change that brings about an increased binding affinity between palonosetron and the 5-HT3 receptor. Increased binding affinity is possibly the result of at least one additional palonosetron molecule binding to the same receptor. Palonosetron also triggers 5-HT3 receptor internalization (29) and inhibits 5-HT3/ NK1 receptor crosstalk (30). Taken together the above moleculat interactions may explain the persistent inhibition of 5-HT3 receptor function and inhibition of substance P responses seen with palosetron. These pharmacological differences help explain the ability of palonosetron, unique among 5-HT3 receptor antagonists, to inhibit delayed emesis (26).
Brain circuitry of nausea
Napadow and colleagues utilized functional magnetic resonance imaging (fMRI) approach evaluated brain activity contributing to and arising from increasing motion sickness (31). They evaluated parametrically increasing brain activity (I) precipitating increasing nausea and (II) following transition to stronger nausea. All subjects demonstrated visual stimulus-associated activation (P<0.01) in primary and extrastriate visual cortices. In subjects experiencing motion sickness, increasing phasic activity preceding nausea was found in the amygdala, putamen, and dorsal pons/locus ceruleus. Increasing sustained responses following increased nausea were found in a broader network including insular, anterior cingulate, orbitofrontal, somatosensory and prefrontal cortices. Sustained anterior insula activation to strong nausea was correlated with midcingulate activation (r=0.87), suggesting a closer linkage between these specific regions within the brain circuitry subserving nausea perception. It appears phasic activation in fear conditioning and noradrenergic brainstem regions may precipitate transition to strong nausea, sustained activation, however, following this transition a broader interoceptive activation of brain regions may occur involving limbic, somatosensory, and cognitive networks, reflecting the multiple dimensions of this aversive commonly occurring symptom (31). A correlation analysis across all brain regions specifically found that subjects who showed greater anterior insula activation following transition to strong nausea also demonstrated greater activation in midcingulate cortex, suggestion a closer linkage between these specific regions (31).
Antiemetic agents
Once that clinicians are more familiar with the receptors associated with nausea/vomiting as well as their locations, related neural circuitry and the affinities of various antiemetic agents to the receptors; they may be better equipped to make decisions regarding the selection of antiemetic agents. Antiemetic agents including: scopolamine, diphenhydramine, promethazine, hydroxyzine, prochlorperazine, droperidol, haloperidol. Metoclopromide, ondansetron (and other 5-HT3 receptor antagonists), aprepitant (and other NK-1 receptor antagonists) and levomepromazine exhibit varying affinities to different receptors. When selecting antiemetic therapy, clinicians should be aware of the receptors being affected and realize that occasionally multi-drug therapy is needed. If multiple agents are used together, they should not be hitting same receptors.
Summary
Nausea and vomiting are distressing symptoms that may significantly detract from overall quality of life and greatly influence a patient’s overall mood and social activities. The treatment of nausea and should be aimed at specific receptors/mediators that appear to be largely contributing to an individual patient’s experience. A greater appreciation of which particular mechanisms are playing a major role for an individual patient may lead to targeted therapies in attempts to eliminate nausea/vomiting, minimize treatmentinduced adverse effects, and optimize patient outcomes.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
Disclosure: The authors has no disclosures and has not published or submitted this manuscript elsewhere.
References
- Melzack R, Rosberger Z, Hollingsworth ML, et al. New approaches to measuring nausea. Can Med Assoc J 1985;133:755-58.
- Donnerer J. The Emetic Reflex Arc. In: Donnerer J. eds. Antiemetic Therapy. Basel, Switzerland: S. Karger AG, 2003:1-10.
- Smith HS. A receptor-based paradigm of nausea and vomiting. Journal of Cancer Pain & Symptom Palliation 2005; 1:11-23.
- Melzack R, Torgerson WS. On the language of pain. Anesthesiology 1971;34:50-9.
- Zhou Q, O’Brien B, Soeken K. Rhodes Index of Nausea and Vomiting--Form 2 in pregnant women. A confirmatory factor analysis. Nurs Res 2001;50:251-7.
- Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control. Experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer 2003;39:1395-401.
- Grunberg SM, Boutin N, Ireland A, et al. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer 1996;4:435-9.
- Terauchi F, Nagashima T, Iwaki S, et al. Evaluation of QOL with orally disintegrating antiemetic tablets in outpatient chemotherapy. Gan To Kagaku Ryoho 2003;30:1465-71.
- Ishihara Y, Nukariya N, Kobayashi K, et al. The development of a new QOL Questionnaire on chemotherapy - induced emesis and vomiting-- investigation of reliability and validity. Group for Investigation of QOL Questionnaire for Anti-Emetics Used in Cancer Chemotherapy. Joint Research Group for Tropisetron Double-Blind Comparative study. Gan To Kagaku Ryoho 1996;23:745-55.
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634-46.
- Borison HL, Wang SC. Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol 1949;12:305-13.
- Wang SC, Borison HL. The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am J Physiol 1951;166:712-7.
- Miller AD, Wilson VJ. ‘Vomiting center’ reanalyzed: an electrical stimulation study. Brain Res 1983;270:154-8.
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol 2002;2:650-6.
- Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 2003;88:4696-701.
- Gaskin FS, Farr SA, Banks WA, et al. Ghrelin-induced feeding is dependent on nitric oxide. Peptides 2003;24:913-8.
- Hermann GE, Tovar CA, Rogers RC. TNFalphastimulation of cFos-activation of neurons in the solitary nucleus is suppressed by TNFR:Fc adsorbant construct in the dorsal vagal complex. Brain Res 2003;976:69-74.
- Furukawa N, Hatano M, Fukuda H, et al. Non-N-methyl- D-aspartate receptors may mediate the transmission of emetic signals between visceral vagal afferents and the solitary nucleus in dogs. Neurosci Lett 1998;258:53-6.
- Vrang N, Phifer CB, Corkern MM, et al. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 2003;285:R470-8.
- Saito R, Suehiro Y, Ariumi H, et al. Anti-emetic effects of a novel NK-1 receptor antagonist HSP-117 in ferrets. Neurosci Lett 1998;254:169-72.
- Fukuda H, Koga T, Furukawa N, et al. The Site of the Antiemetic Action of NK1 Receptor Antagonists. In: Donnerer J. eds. Antiemetic Therapy. Basel, Switzerland: S. Karger AG, 2003:33-77.
- Hesketh PJ, Gandara DR. Serotonin antagonists: a new class of antiemetic agents. J Natl Cancer Inst 1991;83:613-20.
- Freeman AJ, Cunningham KT, Tyers MB. Selectivity of 5-HT3 receptor antagonists and anti-emetic mechanisms of action. Anticancer Drugs 1992;3:79-85.
- Donnerer J. Beubler E. 5-HT3 Receptor Antagonists in Antiemetic Therapy. In: Donnerer J. eds. Antiemetic Therapy. Basel, Switzerland: S. Karger AG, 2003:22-32.
- del Giglio A, Soares HP, Caparroz C, et al. Granisetron is equivalent to ondansetron for prophylaxis of chemotherapy-induced nausea and vomiting: results of a meta-analysis of randomized controlled trials. Cancer 2000;89:2301-8.
- Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2012;684:1-7.
- Tattersall FD, Rycroft W, Francis B, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology 1996;35:1121-9.
- Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 2008;107:469-78.
- Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 2010;626:193-9.
- Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 2010;335:362-8.
- Napadow V, Sheehan JD, Kim J, et al. The Brain Circuitry Underlying the Temporal Evolution of Nausea in Humans. Cereb Cortex 2012. [Epub ahead of print].