The impact of psychosocial intervention on survival in cancer: a meta-analysis
Introduction
Cancer is a leading global cause of death and represents an important public health problem. In 2012, there were an estimated 14.1 million new cancer cases and 8.2 million deaths due to cancer worldwide (1). In addition to a variety of physical symptoms, cancer and its treatments are often associated with significant psychosocial effects, including disruption to social, physical, and cognitive functioning (2-5). Studies show that about one-third of cancer patients are affected by long-term clinical anxiety and depression compared to only about one-fifth of the general population (6-8). To make matters worse, about 40% of young adult cancer patients are unsatisfied with their counseling and psychosocial support (9).
Since the 1980s, a wide variety of psychosocial interventions have been used to treat pain and mood symptoms in cancer patients (10-13). These interventions typically include one or a combination of the following: (I) cognitive-existential group therapy (CEGT); (II) cognitive-behavioral therapy (CBT); (III) supportive-expressive group therapy; and (IV) psychoeducational therapy. Initial studies demonstrated that psychosocial interventions can prolong survival (14,15); however, later studies reported conflicting results regarding the survival benefit of these interventions (16-19). Two independent meta-analyses in 2004 by Chow et al. (20) and Smedslund and Ringdal (21) were conducted to determine the pooled treatment effects of psychosocial interventions on overall survival. Both studies failed to detect a survival difference between intervention and control groups. More recently, a meta-analysis by Xia et al. (22) studied fifteen randomized controlled trials (RCTs) published between 1989 and 2009 and compared survival outcomes at one, two, four, and six years following psychosocial interventions. In contrast to previous analyses, Xia et al. found a significant survival difference at two years of follow-up (RR =0.85; 95% CI, 0.75–0.96; P=0.01). Furthermore, subgroup analysis of seven RCTs exceeding 30 hours in psychosocial treatment revealed a decrease in all RRs and yielded a significant survival benefit in the first two years following intervention.
While the mechanism behind the effect of psychosocial interventions remains unclear, several theories have been proposed. The primary rationale behind CEGT, CBT, and supportive-expressive therapy is to reduce the anxiety and depression associated with cancer treatments; these negative emotional states are believed to impact survival by suppressing immune and neuroendocrine systems (23,24). Patients are also taught problem-solving skills, cognitive flexibility, and relaxation techniques to better cope with stressful situations. Furthermore, psychoeducational therapies have been used to improve patient engagement and compliance in the course of their treatment (25,26).
The management and prognosis of cancer has changed radically over the past few decades and additional RCTs have been published recently (27-31). To our knowledge, there are no reviews that specifically examine recently published RCTs to determine the impact of psychosocial interventions on survival outcomes in cancer patients. The purpose of this meta-analysis is to synthesize evidence from the most recent literature on the survival benefit associated with psychosocial interventions among cancer patients.
Methods
Search strategy
Eligible studies were identified by searching Ovid MEDLINE (2004–May week 3 2015), EMBASE Classic and EMBASE (2004–2015 week 21), and Cochrane Central Register of Controlled Trials (January 2004–April 2015). References of included articles were also screened to identify additional eligible trials. The search algorithm included the following medical subject headings and keywords: (neoplasm OR cancer OR carcinoma) AND (psychotherapy OR psychosocial OR group therapy OR social work OR psychiatric OR counseling OR psychological techniques OR psychoanalytic interpretation OR mental health services).
Inclusion criteria
Articles were eligible for inclusion if they (I) involved an RCT study design; (II) included adult cancer patients; (III) compared one or more groups receiving a psychosocial intervention to a control group receiving an alternate intervention; or no intervention; (IV) provided relevant survival outcomes and/or Kaplan-Meier survival curves. Authors of studies without Kaplan-Meier survival curves and no relevant data were contacted to obtain available survival data. Studies were excluded if they were duplicates, non-English studies, non-original studies, non-clinical trials, case reports or small case series (<5 patients).
Study selection
Two reviewers (WW Fu and A Agarwal) independently screened studies identified for inclusion and determined study eligibility. Disagreements were resolved by consultation from a third opinion (M Popovic).
Data collection and statistical analysis
Data were systematically extracted and tabulated in a standardized database. Extracted variables included the number of patients randomized to intervention or control groups, type of cancer, type of intervention, duration of follow-up, and survival rates at one, two, and four years. Whenever possible, the raw value for the survival rate was recorded. When these rates were unavailable, overall survival rates were estimated from survival curves. To compute survival rates, the cumulative survival was identified at one, two, and four years, and multiplied by the number of patients randomized to each group to estimate the number of survivors.
Pooled treatment effects on survival were compared between intervention and control groups for all cancer patients. A subgroup analysis for primary breast cancer patient-specific trials was conducted as previous studies have shown that these patients live longer compared to cancer patients with metastatic disease from other primary sites (20). Additional subgroup analyses comparing group and individually-delivered psychosocial interventions were conducted, given substantial controversy in the literature regarding their relative survival benefits (30). Tests of statistical heterogeneity using the I2 statistic were applied to assess the extent of observed variability in results between trials.
Risk of bias was assessed using the Cochrane risk of bias tool (32). The tool evaluates methodological quality of the trials based on random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. We evaluated incomplete outcome data by determining the number of patients excluded due to loss at follow-up or missing patient data. Trials were assessed as low risk of attrition bias if less than 20% of patient data were excluded and if similar proportions were excluded from both arms.
Data from each trial were pooled and analyzed using Review Manager (version 5.3) by the Cochrane Collaboration (Oxford, England). The random effects model was applied using the Mantel-Haenszel method to generate risk ratios (RR) estimates with their accompanying 95% confidence intervals (CI).
Results
Literature search
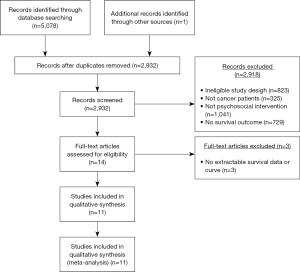
As shown in Figure 1, the search strategy identified a total of 5,080 articles, of which 14 trials were eligible for further review. Among the 14 trials, one of the trials was excluded because it was missing extractable survivable data. Overall, 13 trials met the final inclusion criteria and were included in the meta-analysis (23,27-31,33-39). Table 1 lists studies that exclusively examined patients with breast cancer. Table 2 lists studies that examined patients with other types of cancer or a combination of cancers.

Full table
Full table
Study characteristics
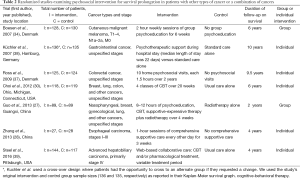
The thirteen included trials contained a total of 2,632 patients, with 1,362 patients randomized to the intervention group and 1,270 patients to the control cohort. Six studies exclusively examined breast cancer patients (23,29,31,33,35,36). Two other trials studied patients with a variety of cancer types including nasopharyngeal, gynecological, breast, lung, colon, and others (27,30). The remaining five trials included patients with cutaneous melanoma (34), esophageal carcinoma (28), colorectal cancer (37), gastrointestinal cancer (38), and hepatobiliary carcinoma (39). Studies were conducted in five countries—specifically, the United States (23,30,31,36,39), Denmark (29,34,37), Australia (33,35), China (27,28), and Germany (38). The intervention arm received psychosocial interventions including CBT (30), small group psychoeducation (34), psychotherapy (23), psychosocial visits (37), or a combination of interventions (27-29,31,33,35,36,38,39). Control arms received usual care (30,38,39), no intervention (28,29,34,37), radiotherapy only (27) relaxation therapy only (33,35), assessment only (23,31) or education only (36). In total, eight of the trials involved group-delivered interventions (23,27,29,31,33-36) while five trials (23,28,30,37-39) involved individually-delivered interventions. One- and two-year survival data was available in all thirteen trials (23,27-31,33-39) and four-year survival data was available in twelve trials (23,28-31,33-39).
Risk of bias assessment
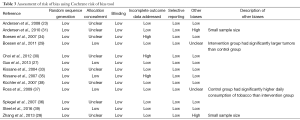
Table 3 reports risk of bias assessments for each trial. All studies demonstrate adequate random sequence generation. However, seven trials had unclear or missing classifications regarding allocation concealment (23,28,30,33,34,36,38). Due to the nature of the interventions, blinding was not applicable in any of the studies (23,27-31,33-39). With respect to incomplete outcome data, ten of the studies (23,27-29,31,33,36-39) were classified as low risk and the remaining three (27,34,35) were deemed high risk for attrition bias. Selective reporting of outcomes was not found to be a source of bias; all trials reported relevant survival outcomes as described in their methods (23,27-31,33-39). Finally, four trials were assessed with unclear or high risk for other biases (27-29,37); two studies had small sample sizes as a potential source of sampling bias (28,31), and two other studies reported differences in patient baseline characteristics between intervention and control groups (29,37).
Full table
Comparison of survival outcomes between psychosocial intervention and control groups
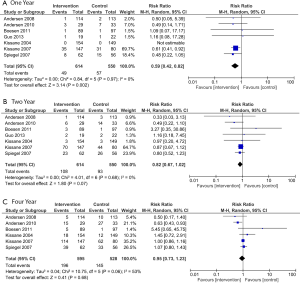
Among all cancer patients, there was a statistically significant improvement in overall survival favouring the psychosocial intervention group at one year (RR =0.82; 95% CI, 0.67–1.00; P=0.04; Figure 2A) and two years (RR =0.86; 95% CI, 0.78–0.95, P=0.003; Figure 2B) but no significant difference at four years (RR =0.94; 95% CI, 0.85–1.04, P=0.24; Figure 2C). The test for statistical heterogeneity was not significant at one, two, or four years (P=0.20, 0.62, and 0.12, respectively).

In breast cancer-only trials, there was a significant improvement in overall survival at one year favoring the psychosocial intervention group (RR =0.59; 95% CI, 0.42–0.82; P=0.002; Figure 3A) but no significant difference at two (RR =0.82; 95% CI, 0.67–1.02, P=0.07; Figure 3B) or four years (RR =0.95; 95% CI, 0.73–1.23; P=0.68; Figure 3C). The test for statistical heterogeneity was not significant at one, two, or four years (P=0.97, 0.68, and 0.06, respectively).
Comparison of group- and individually-delivered Interventions
Among group-delivered intervention trials, there was a significant improvement in overall survival at one-year favoring the psychosocial intervention group (RR =0.57; 95% CI, 0.41–0.79; P=0.0008; Figure 4A), but no difference at two years (RR =0.84; 95% CI, 0.68–1.02; P=0.08; Figure 4B) or four years (RR =0.94; 95% CI, 0.75–1.20; P=0.64; Figure 4C). The test for statistical heterogeneity was not significant at one, two, or four years (P=0.75, 0.69, and 0.10, respectively).
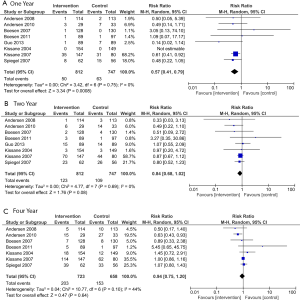
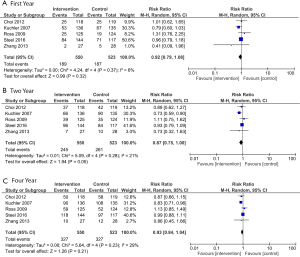
In contrast, among individually-delivered trials, there was no significant difference in overall survival between intervention and control groups at one year (RR =0.92; 95% CI, 0.79–1.08; P=0.32; Figure 5A), two years (RR =0.87; 95% CI, 0.75–1.00; P=0.05; Figure 5B), or four years (RR =0.93; 95% CI, 0.84–1.04; P=0.21; Figure 5C). The test for statistical heterogeneity was not significant at one, two, or four years (P=0.37, 0.28, and 0.23, respectively).
Discussion
The survival benefit of psychosocial interventions in RCTs of cancer patients remains controversial. Previous meta-analyses by Chow et al. (20) and Smedslund and Ringdal (21) failed to detect a significant difference in overall survival rates between intervention and control groups. Chow et al. (20) examined eight RCTs between 1996 and 2002 and found no statistically significant difference in one- and four-year survival (RR =0.94; 95% CI, 0.72–1.22; P=0.6) and (RR =0.93; 95% CI, 0.77–1.13; P=0.5), respectively. Subgroup analysis of four trials containing 511 patients with breast cancer also showed no survival difference at one and four years (RR =0.87; 95% CI, 0.67–1.14; P=0.3) and (RR =0.91; 95% CI, 0.76–1.10; P=0.3), respectively. Smedslund et al. (21) studied 13 articles published between 1989 and 2003 and similarly reported no survival advantage of psychosocial interventions (hazard ratio =0.77; 95% CI, 0.56–1.06; P=0.1). More recently, Xia et al. (22) compared survival rates at one, two, four, and six years with a total of fifteen RCTs, and only reported a significant survival benefit for the psychosocial intervention group at two years of follow-up (RR =0.85; 95% CI, 0.75–0.96; P=0.01). However, subgroup analysis of articles studying interventions with at least 30 hours of treatment revealed a survival advantage at one and two years (RR =0.69; 95% CI, 0.55–0.87; P=0.002) and (RR =0.82; 95% CI, 0.71–0.95; P=0.007), respectively.
The present study examined thirteen RCTs and compared pooled treatment effects on one, two and four-year survival. In contrast to previous studies, the present study showed that psychosocial interventions conferred a short-term survival benefit at one and two years of follow-up in the main analysis. The subgroup analysis of breast cancer showed that psychosocial interventions only had a survival benefit at one year. These discrepancies can be explained by differences in the time period, geographical location, patient characteristics, types of psychosocial interventions, and a relatively small number of studies.
The present study also found that there was no significant survival difference at four years after the intervention, a finding that is consistent with previous meta-analyses (20-22). There are several potential explanations for the lack of statistical significance in the 4-year survival endpoint. First, nearly all of the psychosocial interventions in the included studies lasted one year or less; therefore, the benefits of these interventions may have diminished after the treatment period (23,27-31,34,35,38). Second, contamination bias may exist as patients are often disappointed when they are assigned to the control group and may actively seek psychosocial interventions outside of the trial. This would effectively dilute the impact of psychosocial interventions between the intervention and control groups. In fact, Spiegel et al. (36) found that 43% of control group patients actively joined other social cancer support groups.
Subgroup analyses were also performed to compare survival outcomes between group- and individually-delivered psychosocial interventions. Group-delivered therapies are generally considered more effective than individually-delivered interventions for treating depressive and anxiety symptoms (40-42). Furthermore, studies (43,44) have shown that group-delivered interventions may also be more time-and-cost-efficient than individually-delivered interventions, although the literature is still unclear about which type of therapy is more beneficial for survival outcomes (42). The subgroup analysis found that group-delivered interventions have a significant short-term survival benefit, while individually-delivered interventions show no significant survival benefit.
The present study is subject to certain limitations. The systematic search yielded only 13 relevant clinical trials in the past 10 years, highlighting the paucity of research in this area and the need for additional studies. Furthermore, while statistical heterogeneity was absent, inter-study clinical heterogeneity was more apparent as there were different types of interventions and diverse patient populations (23,30,38). Statistical heterogeneity refers to variability in the intervention effects from the evaluated studies, while clinical heterogeneity refers to variability in the participants, interventions, and outcomes of the evaluated studies. In this meta-analysis, clinical heterogeneity represented a major challenge in terms of synthesizing meaningful conclusions based on pooled analysis. For instance, the present study included trials that examined a variety of psychosocial interventions, including individually-delivered cognitive behavioral therapies (30,34), group supportive-expressive therapies (36,37), and various combinations of psychoeducational and group cognitive therapies (29). In addition, the patients in these trials were characterized by different cancer sites or variable stages of cancer which may have influenced how they responded to the interventions and thus produced biased survival outcomes. For example, Küchler et al. (38) suggested that metastatic or advanced cancer patients may have progressed too far in their disease for psychotherapeutic treatments to yield a substantial impact on survival outcomes relative to patients with early stage disease.
However, because these diverse patient populations were often lumped together in trials and analyzed aggregately in their survival outcomes, it was difficult to isolate the impact of individual cancer stages or cancer sites on survival.
Furthermore, this meta-analysis only incorporated RCTs to minimize differences in baseline demographics between comparators. However, two included RCTs reported variations in patient baseline characteristics which may have influenced the results (29,37). For instance, Ross et al. (37) reported that patients in the control group had a significantly higher daily consumption of tobacco than patients in the intervention group, while Boesen et al. (29) indicated that women in the intervention group had significantly larger tumours than those in the control group.
Kissane et al. (35) also suggested that psychosocial interventions may not appeal to all patients, especially to those who are more distressed or overburdened with treatment complications. These patients are more likely to be forced to delay or withdraw from psychosocial treatments, thereby limiting their participation in these studies and creating a biased sample. On the other hand, patients with lower levels of distress may feel less burdened and more likely to participate in psychosocial interventions. This may have been the case in two studies (29,34) where patients reported relatively low levels of baseline distress. Boesen et al. (29) reasoned that these patients had less room for psychological improvement (i.e., “ceiling effect”) and thus were less sensitive in showing survival improvements. Moreover, data from different studies were pooled irrespective of whether survival was collected as an a priori or post-hoc outcome. Publication bias may be further introduced due to studies that were unpublished because of negative findings or because they were rejected for publication.
While some critics have argued against the efficacy of psychosocial intervention as well as the investment of resources towards a large-scale RCT examining survival outcomes, severe limitations in sample size and between-study heterogeneity among previously-conducted RCTs highlight the need for additional studies with larger, more homogenous patient populations and study characteristics, and examination of long-term outcomes (45).
Conclusions
This meta-analysis of the recent literature demonstrates a significant survival benefit of psychosocial interventions among cancer patients at one and two years following intervention, but a non-significant survival difference at four years relative to controls. Future studies with larger sample sizes, longer follow-up and more homogenous protocols and study populations are needed to validate these results and clarify the long-term survival benefit of these interventions. More comprehensive analyses are also warranted to elucidate differences in outcomes between group- and individually-delivered interventions. Until larger, more comprehensive studies are available to dispute the efficacy of psychosocial interventions, these interventions should continue to be considered in the management of cancer patients given their potential survival benefit. Importantly, clinicians should recognize that long-term treatment may be needed to confer sustained improvement in survival outcomes.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zabora J. The prevalence of psychological distress by cancer site. Psychooncology 2001;10:19-28. [Crossref] [PubMed]
- Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer 2004;90:2297-304. [PubMed]
- Meyerowitz BE. Psychosocial correlates of breast cancer and its treatments. Psychol Bull 1980;87:108-31. [Crossref] [PubMed]
- Spencer S, Carver CS, Price AA. Psychological and social factors in adaptation. In Psycho-oncology. In: Holland JC, editor. Psycho-oncology. New York: Oxford University Press, 1998:211-22.
- Maguire P. Managing psychological morbidity in cancer patients. Eur J Cancer 2000;36:556-8. [PubMed]
- Sukantarat K, Greer S, Brett S, et al. Physical and psychological sequelae of critical illness. Br J Health Psychol 2007;12:65-74. [Crossref] [PubMed]
- Mehnert A, Brähler E, Faller H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol 2014;32:3540-6. [Crossref] [PubMed]
- Zebrack BJ, Corbett V, Embry L, et al. Psychological distress and unsatisfied need for psychosocial support in adolescent and young adult cancer patients during the first year following diagnosis. Psychooncology 2014;23:1267-75. [Crossref] [PubMed]
- Ferlic M, Goldman A, Kennedy BJ. Group counseling in adult patients with advanced cancer. Cancer 1979;43:760-6. [Crossref] [PubMed]
- Gustafson J, Whitman H. Towards a balanced social environment on the oncology service. Soc Psychiatry 1978;13:147-52. [Crossref]
- Wood PE, Milligan M, Christ D, et al. Group counseling for cancer patients in a community hospital. Psychosomatics 1978;19:555-61. [Crossref] [PubMed]
- Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med 1983;45:333-9. [Crossref] [PubMed]
- Spiegel D, Bloom JR, Kraemer HC, et al. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989;2:888-91. [Crossref] [PubMed]
- Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry 1993;50:681-9. [Crossref] [PubMed]
- Ilnychyj A, Farber J, Cheang J, et al. A randomized controlled trial of psychotherapeutic intervention in cancer patients. Ann R Coll Physicians Surg Can 1994.93-6.
- Cunningham AJ, Edmonds CV, Jenkins GP, et al. A randomized controlled trial of the effects of group psychological therapy on survival in women with metastatic breast cancer. Psychooncology 1998;7:508-17. [Crossref] [PubMed]
- Edelman S, Lemon J, Bell DR, et al. Effects of group CBT on the survival time of patients with metastatic breast cancer. Psychooncology 1999;8:474-81. [Crossref] [PubMed]
- Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001;345:1719-26. [Crossref] [PubMed]
- Chow E, Tsao MN, Harth T. Does psychosocial intervention improve survival in cancer? A meta-analysis. Palliat Med 2004;18:25-31. [Crossref] [PubMed]
- Smedslund G, Ringdal GI. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. J Psychosom Res 2004;57:123-31; discussion 33-5. [Crossref] [PubMed]
- Xia Y, Tong G, Feng R, et al. Psychosocial and Behavioral Interventions and Cancer Patient Survival Again: Hints of an Adjusted Meta-Analysis. Integr Cancer Ther 2014;13:301-9. [Crossref] [PubMed]
- Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer 2008;113:3450-8. [Crossref] [PubMed]
- Steel JL, Nadeau K, Olek M, et al. Preliminary results of an individually tailored psychosocial intervention for patients with advanced hepatobiliary carcinoma. J Psychosoc Oncol 2007;25:19-42. [Crossref] [PubMed]
- Jacobs C, Ross RD, Walker IM, et al. Behavior of cancer patients: a randomized study of the effects of education and peer support groups. Am J Clin Oncol 1983;6:347-53. [Crossref] [PubMed]
- Richardson MA, Post-White J, Grimm EA, et al. Coping, life attitudes, and immune responses to imagery and group support after breast cancer treatment. Altern Ther Health Med 1997;3:62-70. [PubMed]
- Guo Z, Tang HY, Li H, et al. The benefits of psychosocial interventions for cancer patients undergoing radiotherapy. Health Qual Life Outcomes 2013;11:121. [Crossref] [PubMed]
- Zhang XD, Zhao QY, Fang Y, et al. Perioperative comprehensive supportive care interventions for chinese patients with esophageal carcinoma: a prospective study. Asian Pac J Cancer Prev 2013;14:7359-66. [Crossref] [PubMed]
- Boesen EH, Karlsen R, Christensen J, et al. Psychosocial group intervention for patients with primary breast cancer: a randomised trial. Eur J Cancer 2011;47:1363-72. [Crossref] [PubMed]
- Choi J, Kuo CW, Sikorskii A, et al. Cognitive behavioral symptom management intervention in patients with cancer: survival analysis. Support Care Cancer 2012;20:1243-50. [Crossref] [PubMed]
- Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res 2010;16:3270-8. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Kissane DW, Love A, Hatton A, et al. Effect of cognitive-existential group therapy on survival in early-stage breast cancer. J Clin Oncol 2004;22:4255-60. [Crossref] [PubMed]
- Boesen EH, Boesen SH, Frederiksen K, et al. Survival after a psychoeducational intervention for patients with cutaneous malignant melanoma: a replication study. J Clin Oncol 2007;25:5698-703. [Crossref] [PubMed]
- Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology 2007;16:277-86. [Crossref] [PubMed]
- Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer 2007;110:1130-8. [Crossref] [PubMed]
- Ross L, Frederiksen K, Boesen SH, et al. No effect on survival of home psychosocial intervention in a randomized study of Danish colorectal cancer patients. Psychooncology 2009;18:875-85. [Crossref] [PubMed]
- Küchler T, Bestmann B, Rappat S, et al. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol 2007;25:2702-8. [Crossref] [PubMed]
- Steel JL, Geller DA, Kim KH, et al. A web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kissane D. Beyond the psychotherapy and survival debate: the challenge of social disparity, depression and treatment adherence in psychosocial cancer care. Psychooncology 2009;18:1-5. [Crossref] [PubMed]
- Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: results of two meta-analyses. Br J Cancer 1999;80:1770-80. [Crossref] [PubMed]
- Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology 2013;22:1457-65. [Crossref] [PubMed]
- Hoffman CJ, Ersser SJ, Hopkinson JB, et al. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol 2012;30:1335-42. [Crossref] [PubMed]
- Cramer H, Lauche R, Paul A, et al. Mindfulness-based stress reduction for breast cancer-a systematic review and meta-analysis. Curr Oncol 2012;19:e343-52. [Crossref] [PubMed]
- Coyne JC, Stefanek M, Palmer SC. Psychotherapy and survival in cancer: the conflict between hope and evidence. Psychol Bull 2007;133:367-94. [Crossref] [PubMed]


