Manipulating the Hippo-Yap signal cascade in stem cells for heart regeneration
Introduction
Ischemic heart disease remains the leading cause of death and disability in the U.S. Although the associated acute coronary syndrome can be attenuated using pharmacotherapy or angioplasty procedures, the heart gradually deteriorates ultimately leading to the heart failure as a consequence of lost cardiomyocytes and pathological remodeling. Stem cell therapy is currently a focus of intensive investigation in view of the fact that it has the potential to regenerate or repair infarcted tissue. Many techniques have adopted this promising therapeutic strategy to treat ischemic heart diseases through intravascular infusion, intramyocardial injection, or bio-membrane/patch-based delivery of cells to the myocardium. Randomized and controlled clinical trials have also demonstrated that stem cell therapy can improve the recovery of cardiac function in patients after acute myocardial infarction. However, decreased cell survival and engraftment efficiency are two major pitfalls that can compromise efficacy. The vast majority of transplanted cells are lost within days in situ due to the anoxic environment inside heart tissue, and the formation of fibrosis hinders the migration of transplanted cells to the site of damaged tissue.
Hippo complexes, also known as Salvador/Warts/Hippo (SWH) pathway, were originally identified as a signal cascade controlling Drosophila organ size through the regulation of cell proliferation and apoptosis (1). Orthologs of Hippo components have subsequently been recognized in mammals, and this highly conserved signal axis appears to be crucial in maintaining organ development in vertebrates. In vivo experimental research has shown that temporal activation of the principal Hippo effector Yap (Yes-associated protein) led to massive hepatomegaly (2). A recent study also demonstrated that Yap serves as a critical downstream effector of the Hippo pathway controlling embryonic heart size, in which constitutive activation of Yap significantly enhanced cardiomyocyte number and increased heart size while ablation of Yap resulted in myocardial hypoplasia and mouse lethality during the embryonic stage (3).
Tissue regeneration is a repair and renewal process, which is an essential and fundamental feature in multicellular organisms in response to injury. It is Hippo’s potency in coordinating cell proliferation, differentiation, and tissue homeostasis that has encouraged further research into regulation of this signaling pathway as a potential therapeutic strategy in regenerative medicine. Using Xenopus as a model organism, both Tead4 and Yap1 have been documented as Hippo pathway transcriptional regulators required for general vertebrate epimorphic regeneration, as well as for organ size control in appendage regeneration. These Hippo transcription factors regulate the growth and fate of tissue cells in Xenopus tadpole tail, including spinal cord, notochord, muscles, and blood vessels, in both time- and position-dependent manner (4). This suggests a precisely programmed regulatory mechanism that has contributed to the development of appropriately sized three-dimensional organs in response to injury. Unfortunately, heart muscles exert feeble regenerative ability in response to a variety of damages, which is one of the primary contributors to heart failure post ischemic injury. As a result, cardiac Hippo signaling (5) has become a focus of research in the field of cardiac regenerative medicine. Pronounced cardiac regeneration has been observed in myocardial infarcted regions when the active form of Yap was constitutively expressed in heart tissue, associated with a default fibrotic response (6). Using Yap gain- and loss-of-function genome-wide analysis and RNA sequencing techniques, the phosphoinositol-3-kinase-Akt pathway was identified as a functional downstream mechanism promoting mitogenic activity and stimulating endogenous cardiomyocyte proliferation in response to the heart tissue injury (7).
With the advent of human induced pluripotent stem cell (iPSC) technology, it is now possible to treat myocardial infarction with autologous iPSC-derived cardiomyocytes. However, induction and acquirement of mature cardiomyocytes from iPSCs and functional integration of these committed differentiated cells into injured myocardium often compromises the translational therapeutic value of this technique in a clinical setting. This article will discuss the role of the Hippo-Yap signaling cascade in cardiogenic differentiation of stem cells and its translational therapeutic value in myocardial infarction treatment.
Core components of the Hippo-Yap signal pathway
The Hippo signaling complex consists of a cluster of cytoplasm-located protein kinases and two major transcription factors associated with correspondent regulators. Enrichment of these pathway components with WW domains and their cognate proline-rich interacting motifs provide an efficient signaling mechanism to sense upstream input and start the downstream output (8). Briefly, Mst can be activated by a variety of stress signals including mechanic stress, extracellular matrix stiffness, cytoskeletal rearrangement, contact inhibition and anoxemia (9). This signal activation can directly modulate mitochondrial function to affect energy metabolism, or Mst can transduce this activation to Lats1/2 (large tumor suppressor kinase) to phosphorylate Yap, which will subsequently be degraded by the ubiquitin proteasome pathway. Contrarily, inhibition of Hippo signals will protect against Yap degradation and promote its nuclear translocation (Figure 1).
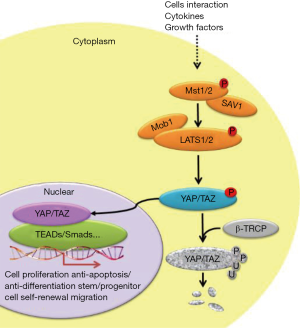
Mammalian sterile 20-like kinase 1 (Mst1)/2 serves as a response kinase
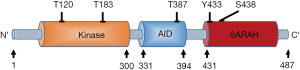
The full-length of Mst1 is composed of N-terminal kinase domain, a C-terminal Sav/Rassf/Hpo (SARAH) domain, with an auto-inhibitory domain (AID) between them. Mst1 activity is regulated by the phosphorylation at threonine residues (T120, T183, T387), tyrosine residue (Y433), and serine residue (S438) (Figure 2). Increased phosphorylation levels at T183 and Y433 will lead to Mst1 activation (10,11), whereas Mst1 activity will be inhibited when it is phosphorylated on T120, T387, and S438 (10,12,13). Mst1 acts as a responsive kinase under stressful conditions to phosphorylate Bcl-xL at Serine14 in the BH4 domain, thereby blockading the Bcl-xL/Bax covalent interactions and subsequently triggering mitochondria-mediated apoptotic death through the activation of Bax (14). Further studies have demonstrated that Mst1 actually serves as a switch between cardiac cell life and death through dual regulation of autophagy and apoptosis (15). Indeed, during cellular stress, the activated Mst1 can phosphorylate Beclin1 at threonine-108, leading to the separation of Beclin-1 from Atg14L-Vps34 complex. The dissociated Beclin1 subsequently competes with Bax to form complexes with Bcl-2, which can suppress autophagy and result in the accumulation of protein aggregates. Unbound Bax will translocate to mitochondria triggering mitochondrial perturbations, leading to the release of cytochrome c and caspase activation resulting in apoptosis (15,16).
Regulation of Lats1/2 kinase activity in Hippo complex
Mammalian Last1/2 kinase is classified as a subgroup of the AGC protein kinase family, which is conserved among eukaryotes. The structure of this serine/threonine kinase features a conserved N-terminal regulatory domain (NTR) and an insert between subdomains of the catalytic kinase domain. It has been earlier reported that Lats locate to the centrosome and negatively regulate cell cycle progression through inhibition of cell cycle controller CDC2 kinase in early mitosis (17). More recent studies have demonstrated this kinase is also involved in the regulation of F-actin binding, cell migration (18), and cell morphology (19). Like other kinases, activation of Lats kinases result from the phosphorylation of serine/threonine residues on the activation segment motif (AS) and a C-terminally located hydrophobic motif (HM) regulated by a group of upstream protein kinases including Mst1/2, Cdk1, Aurora A, CHK1/2, and PKA. Among them, Mst1/2-induced phosphorylation on Thr1079 and Ser909 are essential for maintaining kinase activity, and this activation can be counteracted by PP2A-mediated dephosphorylation within the AS and HM region (20,21). Notably, structural and biochemical investigations strongly indicate that the conserved NTR domain of Lats1/2 also contain a binding motif that can interact with Mob, but it is unclear how this regulatory protein affects Lats activity. One proposed mechanism suggests that Mst1/2 kinases phosphorylate Mob1 and thereby promote the formation of Mob1/Lats complexes, and this covalent binding enables efficient autophosphorylation within the AS domain of Lats (22).
Yap, an effector for the Hippo complex
Yap is a transcriptional co-activator shuttling between the nucleus and cytoplasm, and this spatial alteration is mainly determined by the phosphorylation on residue Serine 127 or Serine 379. In vitro experimental evidence shows that Yap is subjected to cytoplasmic retention and ubiquitin-dependent degradation upon Lats kinase-induced phosphorylation, whereas phosphorylated Yap translocate into the nucleus. The increased amount of Yap can be observed in the nucleus when residue Serine 127 is changed into Alanine, a mutation form that keeps this residue from phosphorylation. Correspondingly, Yap-induced biological effects (such as cell proliferation) are also enhanced to a greater extent in S127A-mutated Yap when compared to wild type Yap. Nuclear-translocated Yap cannot recognize and interact with DNA binding domain per se. Instead, this Hippo effector serves as co-activator along with TAZ to regulate the DNA-binding activity of Tead, a crucial transcriptional factor that triggers proliferative and prosurvival gene progression programs. Tead contains a C-terminal protein binding domain that interacts with the N-terminal of Yap. This is supported by nuclear magnetic resonance (NMR) evidence that the Yap-binding domain of Tead is characterized by an immunoglobulin-like beta-sandwich fold with two extra helix-turn-helix inserts, and this structural feature can precisely recognize and covalently bind to the Tead-binding domain of Yap (23). Correspondingly, Yap wraps around the globular structure of Tead and forms extensive interactions via three highly conserved interfaces (24). Alternatively, Tead contains an N-terminal TEA domain, a DNA binding module that can interact with canonical M-CAT elements to regulate target gene expression. M-CAT sequence motif (5’-TCATTCCT-3’) has been identified in several gene promoters and is the decisive DNA region controlling regulation of cell growth, differentiation, and epithelial-mesenchymal transition. Notably, the enhanced protein-protein interaction between Yap and Tead has been identified as a molecular mechanism contributing to oncogenesis and metastasis (especially hepatocellular carcinoma and gastric cancer (25,26), and pharmacological blockade of Yap-Tead complex formations may be an important novel therapeutic strategy for inhibiting tumor growth (27).
Hippo signals and stems cell pluripotency
Pluripotency, a hallmark characteristic of stem cells, is defined by the dual features of self-renewal and differentiation potential, giving rise to the spectrum of cell types that comprise an organism and holding significant promise in regenerative medicine therapeutics. Studies have confirmed that iPSC techniques can enable somatic cells to acquire pluripotency through genetic reprogramming, providing an opportunity to diagnose and treat malignant disorders by simply replacing dysfunctional cells with iPSC-derived functional counterparts. However, barriers to reprogramming become significant in advanced developed somatic cells, especially under the conditions of aging, metabolism disturbance, and hypoxia. These restraints include genes involved in transcription, chromatin regulation, ubiquitination, dephosphorylation, vesicular transport, and cell adhesion (28). Recent studies reveal the Hippo pathway as an important regulator in reprogramming process.
Comparison of transcriptional profiles between pluripotent and somatic cells has revealed Lats as a negative regulator of pluripotency. Silencing of the endogenous LATS specifically enhances generation of fully reprogrammed iPS cells without accelerating cell proliferation, suggesting that Hippo pathway activation may constitute a barrier to cellular reprogramming (29) and blockade of Hippo signals may promote this process. Correspondingly, constitutive overexpression of Yap5SA, a Yap mutation form which is no longer suppressed by the Hippo pathway, can maintain embryonic stem cell morphology and pluripotency as evidenced by the stable alkaline phosphatase staining and the sustained expressions of Sox2 and Oct4 (30). Several studies have made strides to elucidate the molecular mechanism of Hippo components in maintaining stem cell pluripotency. Proteomics approach has been utilized to identify that the promoter of mesendodermal genes, a group of transcription factors critically involved with self-renewal of undifferentiated embryonic stem cells, is bound by OCT4, NANOG, and SMAD2/3/4. At the pluripotent stage, YAP/TAZ and TEAD act to recruit the NuRD (nucleosome remodeling and deacetylase) complex to bind to a promoter, which subsequently represses mesendodermal gene expression by precluding the transcription factor-induced initiation. On the contrary, YAP/TAZ and TEAD no longer bind to these genes upon the mesendodermal differentiation (31,32). A recent study also revealed that the Hippo pathway members can enrich Sox2 to the inner cell mass (ICM) where it can promote ICM fate in mouse blastocysts (33).
Considered a key component in maintaining pluripotency, the Hippo signal complex has been identified as a therapeutic target that terminates the stemness of tumor cells, and some small molecules [such as verteporfin (VP)] have been designed to inhibit Yap-Tead interaction. VP treatment can disrupt Yap-Tead signaling and downregulate Oct4 expression in hepatoma cells and human retinoblastoma cell lines (34). Interestingly, the Yap-mediated hepatocyte reprogramming has been identified as a mechanism contributing to human hepatocellular carcinoma (HCC) pathogenesis, and the enriched Yap targets have been detected in the aggressive human HCC subtype, which features a proliferative signature and absence of the CTNNB1 mutation. Importantly, targeting Yap with small interfering RNA-lipid nanoparticles can significantly restore hepatocyte differentiation and result in pronounced tumor regression in a genetically engineered mouse HCC model (35). On the other hand, Hippo pathway modulation can regulate stem cell proliferation and maintenance and may be useful therapeutically for tissue repair and regeneration following injury (36).
Hippo-Yap signal in heart development and myocyte maturation
The importance of Yap in regulating heart development has been explored in several studies using gain- and loss-of-function approaches. In one experiment, slower heartbeat and decreased number of cardiac Troponin-positive cardiomyocytes were observed, which consequently resulted in embryonic death in inducible Yap gene mutant embryos. Although cardiac looping and chamber formation was not affected, deletion of Yap diminished the proliferation of cardiomyocytes, leading to a significant reduction in the number of ventricular myocytes when compared with the wild type littermates. Correspondingly, forced expression of YapS112A, a Yap mutant form that is constitutively active and localized to the nucleus, significantly promoted the proliferation of cardiomyocytes in the hearts of transgenic embryos, and YapS112A transgenic mice displayed an abnormally thickened myocardium and expanded trabecular layer compared with that of Yap transgenic mice (3). Compromised cellular phenotype was similarly also observed in Mst deficient embryonic body (Mst−/− EBs), in which beating cell clumps disappeared and the expressions of cardiac progenitor markers (such as Nkx2.5, Tbx5, Mesp1, Isl1 and Baf60c) were significantly suppressed. Further studies revealed that Mst are involved in cardiogenesis with a mechanism that regulates non-canonical Wnt ligands. Expression and secretion of several non-canonical Wnt ligands (such as Wnt2, Wnt2b, and Wnt5a) were reduced in Mst−/− EBs, whereas canonical Wnt ligand genes expression were not affected (37). Numerous studies have provided evidence that Yap is a nexus of multiple signaling pathways in governing cardiac growth and survival. Actually, Yap builds up an interlink among Hippo pathway, Wnt pathway, and the IGF pathway to regulate β-catenin signaling and precisely control cardiac development (3).
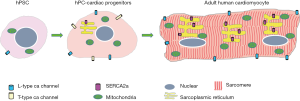
In the process of cardiac differentiation, cardiomyocyte morphology maturation is characterized by enhanced myofibril density and alignment, associated with visible sarcomeres under bright-field microscopy (38). Functional maturation is indicated by increased ion channel expression in the cell membrane, enhanced calcium storage capacity in sarcoplasmic reticulum (SR), high density distribution of adrenergic receptors, and robust contractility. These differences can be revealed in comparisons between human pluripotent cells, pluripotent cell-derived cardiomyocytes, and adult cardiomyocytes (Figure 3) (39). There is no evidence, so far, that Hippo-Yap signals directly regulate the functional maturation of electrophysiology and calcium handling during cardiomyocytes differentiation. However, a most recent study demonstrated this signal pathway is involved in the actin cytoskeletal remodeling with protrusion formation, using the Salvador gene knock out (Salv KO) mouse model and chromatin immunoprecipitations (ChIP) sequencing. Mst1 activation is dependent on the interaction with Salvador. Ablation of Salvador will inhibit the kinase activity of whole Hippo signals, thereby leading to the nuclear accumulation of non-phosphorylated Yap. Actually, Yap-Chip sequencing and mRNA expression profiling in Salv KO hearts revealed that Yap is involved in gene transcription and regulation of Sarcoglycan and Talin2, which can compose the plasmalemmal complexes that link the actin cytoskeleton to the extracellular matrix. Importantly, this was confirmed in mouse ischemic hearts post left anterior descending artery ligation. The greater extent of cytoskeleton rearrangement was observed in Hippo kinase-compromised cardiomyocytes than in wild type counterparts, which enabled the migration of cardiomyocytes into infarct border-zone. Upregulation of Sarcoglycan and Talin2 help Salv KO cardiomyocytes extend sarcomere-filled protrusions into scar tissue in the region of myocardial injury, as evidenced by the appearance of costameres linking the ECM to the actin through the integrin-vinculin-talin complex, which is essential cellular event for heart regeneration (40).
Taken together, functional inhibition Hippo signals can promote cardiac differentiation of stem cells, whereas activation of these kinases impedes the process. In addition, it would be interesting to investigate the involvement of Hippo-Yap in stem cell electrophysiological mutation in future studies.
Hippo-Yap signal and cardiomyocyte proliferation
Adult mammalian cardiomyocytes mostly withdraw from the cell-cycle and therefore do not proliferate. The mammalian heart is normally thought to grow by enlargement without cardiomyocyte proliferation during the postnatal period, thereby restraining the intrinsic regenerative capability of the adult hearts. Interestingly, cytokinesis and mitosis are detectable both in human and murine cardiomyocytes throughout one’s life, and the incidence of these myocytes proliferating events appear more frequent at young age (7,41). Yap’s regulatory effect on cardiomyocytes proliferation has been revealed for the most part through gain- and loss-of-function experiments. Yap deficiency impaired cardiomyocyte proliferation, whereas overexpression promotes cell-cycle activity in cardiomyocytes both in vitro and in infant hearts (42). A recent study using genome-wide screen and high throughput sequencing on Yap-ChIPs indicated that Yap can facilitate Tead binding to the enhancer of Pi3kcb, which encodes p110β as catalytic subunit of phosphoinositol-3-kinase. Particularly, Pik3cb gain-of-function can promote cardiomyocytes proliferation, while ablation of Pik3cb can reduce Yap pro-mitogenic activity (7). Further studies have revealed enhanced phosphorylation of AKT on Serine-473 upon Pi3kcb overexpression, which subsequently triggers degradation of cell-cycle inhibitor P27 and results in the cell proliferation (7). A post-transcriptional regulatory mechanism for Hippo complexes has been recently reported. A microRNA cluster miR302-367 can repress Mst1, Lats, and Mob1b mRNA expressions through their respective 3’-untraslatonal region, which subsequently promotes the nuclear entry of Yap and cardiomyocyte proliferation (43). Indeed, the expression of this microRNA cluster is detected at peak levels at embryonic stage, but disappears at postnatal and adult stages, indicating its importance for cardiomyocytes proliferation during early heart development. Interestingly, postnatal re-expression of miR302-367, as well as systemic delivery of miR302 mimic can reactivate the cell-cycle and increase mitogenic activity in adult cardiomyocytes, reducing scar formation post experimental myocardial infarction (43). These studies indicate that precise targeting on Hippo signals can promote cardiomyocyte proliferation, producing protective and beneficial effects.
Hippo-Yap signal and cardiac stem cell migration
Precise regulation of stem cell movement is crucial for organogenesis of the vertebrate heart during development, and is critical for heart tissue repair after injury (especially in the presence of a fibrotic barrier under hypoxic conditions). Recently, a genetically modified zebrafish system (44) was used to reveal the biological significance of Hippo-Yap complexes in steering cardiac stem cell migration. Temporal analysis of the Yap/Taz-Tead nuclear signal and activity in zebrafish indicated that both are intensified in cardiac precursors and cardiomyocytes, while disruption of Yap/Taz activity lead to a failure of midline migration for cardiac progenitors, resulting in significant cardia bifida (44). It is still unknown which genes are targeted by Yap/Taz to regulate cell movement in cardiac stem cells, but the potential molecular mechanisms have been partially demonstrated in cancer cell studies that include upregulation of genes responsible for chemokine, chemokine receptor, and cytoskeleton reorganization. As an upstream kinase of Yap, Lats1 can steer cell migration independent of the Yap-induced transcription regulatory mechanism. The most recent study on breast cancer cells demonstrated that p53 phosphorylation is reduced after exposure to Lats1 siRNA, which subsequently affects p53 activity with enhanced binding to p52, a member of the NF-κB transcription factor family. This interactome consequently promotes PTGS2 expression levels resulting in increased cell movement and migration (45). Interestingly, many canonical migration pathways, such as epidermal growth factor receptor (EGFR) signaling (46) and HMG-CoA reeducates (HMGCR) cascade (47), can crosstalk with Hippo-Yap to produce synergistic effect on cell migration, revealing the significance of this pathway in regulating cell mobility.
Hippo-Yap signal and mitochondrial biogenesis in cardiac stem cell
Mitochondria occupy approximately one-third of cellular volume in adult cardiomyocytes and play a central role in energy metabolism, and ATP is predominantly generated through oxidative phosphorylation in this cellular powerhouse (48). Therefore, mitochondrial maturation, both in morphology and function, has been recognized as a hallmark event in differentiation of embryonic stem cells and iPS cells. Mst1 is ascertained as an endogenous inhibitor of autophagy (16), which has been recently recognized as a cellular event during mitochondrial biogenesis in myoblast myogenic differentiation (49), indicating that Hippo signaling might be involved in the regulation of mitochondrial maturation in cardiac stem cells maturation. Indeed, the dynamic remodeling of mitochondrial network, including autophagy-induced mitochondrial clearance and optic atrophy 1 (OPA1)-mediated mitochondrial repopulation, facilitates the switch of metabolic mechanism from glycolysis to oxidative phosphorylation upon myogenic differentiation. Upon activation, myoblasts can fuse with other newly differentiated or preexisting myoblasts to constitute myotubes, which is a metabolically active cell type that depends predominantly on oxidative phosphorylation. To meet and maintain this enhanced energetic requirements, the expanded mitochondrial mass and the formation of an intensive mitochondrial network are demanded. Actually, dynamin-1-like protein (DNM1L) is up-regulated but cleared soon after, during the early phase of differentiation. The abrupt increased expression of this GTPase can induce mitochondrial fission to facilitate mitophagy, which will consequently break down the low dense mitochondrial network and recycle the sparsely distributed small size mitochondria. Post the elimination and clearance of most preexisting mitochondria, the brisk up-regulation of mitochondrial fusion protein, OPA1, will lead to the replenishment of high density mitochondrial network via peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PPARGC1A)/PGC-1a-mediated mitochondrial biogenesis. Notably, the autophagic flux in early-phase of myogenic differentiation appeared to be an essential stage for mitochondrial remodeling, since the mitochondrial reconstitution phase won’t occur till mitophagy-mediated clearance has been completed. Both in vitro and in vivo evidences revealed that pharmacological blockade, gene silencing or genetic ablation of essential autophagy components, such as Atg5, BAF and Sqstm1, can significantly inhibit autophagic flux and impair mitochondrial remodeling in myoblasts, and subsequently prevent myogenic differentiation (49). A recent study indicated that Mst1, an autophagy suppressor, are required for the proper cardiac lineage cell development. Although Mst1 deficient embryonic stem cells can differentiate into mesoderm lineage, the further differentiation into cardiac lineage cells is drastically inhibited, suggesting that Mst1 might be involved in the regulation of autophagic flux and dynamic mitochondrial remodeling.
Conclusions and perspective
An accumulating amount of evidence implicates the Hippo-Yap signal axis as an indispensable and paramount mechanism in regulating cardiac development, regeneration, and rejuvenation (50). At the level of mitochondria, upregulating Hippo can modify cardiac response to stress by modulating Mst1 activity, which appears to suppress autophagy and can promote cardiomyocyte apoptosis through phosphorylation and inhibition of Bcl-xL (14,16). Importantly, inhibition of Mst1 activation using its dominant-negative mutation can act as protection against cardiomyopathy by reducing myocyte necrosis and apoptosis (51). The nuclear entry of Yap can be mediated by Mst1 activation through sequential phosphorylation of Hippo components, which will regulate gene transcription by guiding Tead to the corresponding transcription factor binding motif. It has recently become apparent that this coordinated gene transcription regulatory mechanism represents a nodal point in regulation cardiac cell proliferation, differentiation, and regeneration. Indeed, perturbation of Yap-Tead interaction with a blocking peptide significantly inhibits Yap overexpression-induced upregulation of cell-cycle-related genes, such as Aurkb, cdc20, and Ccna2 (42). It is important to note that these studies were performed mainly in mouse models, and Hippo-Yap cascades are seldom studied in specific types of cardiac stem cells, such as cell lineages expressing c-Kit, Sca-1 and MDR-1. In addition, the studies conducted in rodents differ significantly from Hippo-Yap function studies done on humans. Therefore, it remains necessary to investigate the role of Hippo-Yap in cardiogenic differentiation, cardiomyocyte proliferation, and cardiomyocyte regeneration in a clinical setting. Fortunately, recent developments in iPSC technology have provided researchers with a unique platform to assess signaling pathways in specific types of human cells by converting somatic cells into stem-like cells. As such, it would be interesting to investigate the Hippo-Yap signaling complexes in human iPSCs, especially in acquiring pluripotency, cardiogenic differentiation, and functional maturation of cardiomyocytes, which holds great promise for future cardiac regenerative medical therapeutics.
Acknowledgements
Funding: This work was supported by grant from the National Institutes of Health, USA (R01HL110740, and R01HL107957 to Yigang Wang), the grant from National Natural Science Foundation of China (grant No. 81370230 and No. 81570279); and the grant from Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Human Resources and Social Security of China (grant No. Z012013046).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003;114:445-56. [Crossref] [PubMed]
- Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007;130:1120-33. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 2011;4:ra70. [Crossref] [PubMed]
- Hayashi S, Ochi H, Ogino H, et al. Transcriptional regulators in the Hippo signaling pathway control organ growth in Xenopus tadpole tail regeneration. Dev Biol 2014;396:31-41. [Crossref] [PubMed]
- Papizan JB, Olson EN. Hippo in the path to heart repair. Circ Res 2014;115:332-4. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A 2013;110:13839-44. [Crossref] [PubMed]
- Lin Z, Zhou P, von Gise A, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res 2015;116:35-45. [Crossref] [PubMed]
- Salah Z, Aqeilan RI. WW domain interactions regulate the Hippo tumor suppressor pathway. Cell Death Dis 2011;2:e172. [Crossref] [PubMed]
- Zhou X, Wang Z, Huang W, et al. G protein-coupled receptors: bridging the gap from the extracellular signals to the Hippo pathway. Acta Biochim Biophys Sin (Shanghai) 2015;47:10-5. [Crossref] [PubMed]
- Sciarretta S, Zhai P, Maejima Y, et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep 2015;11:125-36. [Crossref] [PubMed]
- Xiao L, Chen D, Hu P, et al. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J Neurosci 2011;31:9611-9. [Crossref] [PubMed]
- Collak FK, Yagiz K, Luthringer DJ, et al. Threonine-120 phosphorylation regulated by phosphoinositide-3-kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-like kinase 1. J Biol Chem 2012;287:23698-709. [Crossref] [PubMed]
- Banga S, Patil GP, Taillie C. Likelihood contour method for the calculation of asymptotic upper confidence limits on the risk function for quantitative responses. Risk Anal 2001;21:613-23. [Crossref] [PubMed]
- Del Re DP, Matsuda T, Zhai P, et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell 2014;54:639-50. [Crossref] [PubMed]
- Dhingra R, Kirshenbaum LA. Mst-1 switches between cardiac cell life and death. Nat Med 2016;19:1367-8. [Crossref] [PubMed]
- Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med 2013;19:1478-88. [Crossref] [PubMed]
- Yabuta N, Mukai S, Okada N, et al. The tumor suppressor Lats2 is pivotal in Aurora A and Aurora B signaling during mitosis. Cell Cycle 2011;10:2724-36. [Crossref] [PubMed]
- Dai X, She P, Chi F, et al. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J Biol Chem 2013;288:34041-51. [Crossref] [PubMed]
- Wada K, Itoga K, Okano T, et al. Hippo pathway regulation by cell morphology and stress fibers. Development 2011;138:3907-14. [Crossref] [PubMed]
- Chan EH, Nousiainen M, Chalamalasetty RB, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005;24:2076-86. [Crossref] [PubMed]
- Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun 2006;345:50-8. [Crossref] [PubMed]
- Hergovich A, Hemmings BA. Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors 2009;35:338-45. [Crossref] [PubMed]
- Tian W, Yu J, Tomchick DR, et al. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci U S A 2010;107:7293-8. [Crossref] [PubMed]
- Li Z, Zhao B, Wang P, et al. Structural insights into the YAP and TEAD complex. Genes Dev 2010;24:235-40. [Crossref] [PubMed]
- Lamar JM, Stern P, Liu H, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A 2012;109:E2441-50. [Crossref] [PubMed]
- Qiao Y, Lin SJ, Chen Y, et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Zhou Z, Hu T, Xu Z, et al. Targeting Hippo pathway by specific interruption of YAP-TEAD interaction using cyclic YAP-like peptides. FASEB J 2015;29:724-32. [Crossref] [PubMed]
- Qin H, Diaz A, Blouin L, et al. Systematic identification of barriers to human iPSC generation. Cell 2014;158:449-61. [Crossref] [PubMed]
- Qin H, Blaschke K, Wei G, et al. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Hum Mol Genet 2012;21:2054-67. [Crossref] [PubMed]
- Lian I, Kim J, Okazawa H, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 2010;24:1106-18. [Crossref] [PubMed]
- Beyer TA, Weiss A, Khomchuk Y, et al. Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep 2013;5:1611-24. [Crossref] [PubMed]
- Mullen AC. Hippo tips the TGF-β scale in favor of pluripotency. Cell Stem Cell 2014;14:6-8. [Crossref] [PubMed]
- Wicklow E, Blij S, Frum T, et al. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet 2014;10:e1004618. [Crossref] [PubMed]
- Brodowska K, Al-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res 2014;124:67-73. [Crossref] [PubMed]
- Fitamant J, Kottakis F, Benhamouche S, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep 2015. [Epub ahead of print].
- Santucci M, Vignudelli T, Ferrari S, et al. The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment. J Med Chem 2015;58:4857-73. [Crossref] [PubMed]
- Li P, Chen Y, Mak KK, et al. Functional role of Mst1/Mst2 in embryonic stem cell differentiation. PLoS One 2013;8:e79867. [Crossref] [PubMed]
- Lundy SD, Zhu WZ, Regnier M, et al. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 2013;22:1991-2002. [Crossref] [PubMed]
- Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 2013;31:829-37. [Crossref] [PubMed]
- Morikawa Y, Zhang M, Heallen T, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal 2015;8:ra41. [Crossref] [PubMed]
- Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 2013;110:1446-51. [Crossref] [PubMed]
- von Gise A, Lin Z, Schlegelmilch K, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A 2012;109:2394-9. [Crossref] [PubMed]
- Tian Y, Liu Y, Wang T, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med 2015;7:279ra38.
- Miesfeld JB, Link BA. Establishment of transgenic lines to monitor and manipulate Yap/Taz-Tead activity in zebrafish reveals both evolutionarily conserved and divergent functions of the Hippo pathway. Mech Dev 2014;133:177-88. [Crossref] [PubMed]
- Furth N, Bossel Ben-Moshe N, Pozniak Y, et al. Down-regulation of LATS kinases alters p53 to promote cell migration. Genes Dev 2015;29:2325-30. [Crossref] [PubMed]
- He C, Mao D, Hua G, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med 2015;7:1426-49. [Crossref] [PubMed]
- Qiu Z, Yuan W, Chen T, et al. HMGCR positively regulated the growth and migration of glioblastoma cells. Gene 2016;576:22-7. [Crossref] [PubMed]
- Cai WF, Kang K, Huang W, et al. CXCR4 attenuates cardiomyocytes mitochondrial dysfunction to resist ischaemia-reperfusion injury. J Cell Mol Med 2015;19:1825-35. [Crossref] [PubMed]
- Sin J, Andres AM, Taylor DJ, et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016;12:369-80. [Crossref] [PubMed]
- Lin Z, Pu WT. Harnessing Hippo in the heart: Hippo/Yap signaling and applications to heart regeneration and rejuvenation. Stem Cell Res 2014;13:571-81. [Crossref] [PubMed]
- Lee GJ, Yan L, Vatner DE, et al. Mst1 inhibition rescues β1-adrenergic cardiomyopathy by reducing myocyte necrosis and non-myocyte apoptosis rather than myocyte apoptosis. Basic Res Cardiol 2015;110:7. [Crossref] [PubMed]


