Enteral versus parenteral nutrition in cancer patients: evidences and controversies
Introduction
The debate over the use of enteral nutrition (EN) and parenteral nutrition (PN) is an old but evergreen and hot topic. Since many years, studies comparing EN and PN have been a pivotal ‘leitmotif’ in the published literature on artificial nutrition (AN). Moreover, during the last 15 years conflicting results have been coming out from randomized controlled trials (RCT) and meta-analysis regarding the benefits of EN versus PN in intensive care unit (ICU), surgical or cancer populations. Specifically, the issue of the route for delivering AN in cancer patients is important and attractive. Thus, I read the paper of Chow et al. (1) with interest, and I compliment with the authors for facing such a controversial topic. As in many other papers of this type, any conclusion is hardly generalizable to the overall cancer population. Actually, there is a background misunderstanding in this debate; specifically, that EN and PN are competitors in the choice of the route for delivering nutrition support in cancer patients. Conversely, EN and PN have specific indications and contraindications.
This review has the purpose to discuss the indications and complications as well as pros and cons of EN and PN in cancer patients, the crucial role of nutrition support in oncology patients during anticancer treatments and throughout the course of disease, and, finally, the role of AN in advanced cancer patients.
Parenteral nutrition (PN)
In cancer patients undergoing chemotherapy, the guidelines (2-4) recommend to initiate EN if oral food intake remains insufficient despite dietary counseling and oral nutritional supplementation, and PN if EN is not sufficient or feasible. Moreover, in patients with chronic insufficient oral food intake and/or uncontrollable malabsorption due to partial obstruction of the gastrointestinal (GI) tract, home AN (HAN) is recommended. Incurable cancer patients may enter a home PN (HPN) program if they are unable to meet their nutritional requirements by oral or enteral route and there is a risk of death due to malnutrition (5).
Prescribing, compounding, and dispensing PN in cancer patients is a multidisciplinary process involving many healthcare professionals: physicians, dieticians, nurses, and pharmacists, usually as members of a Nutrition Support Team (6). The prescriber of PN should be well versed in the appropriate indication for PN, basic in sterility and infection control, vascular access devices (peripheral and central) and their associated complications. An appropriate use of PN can maximize its clinical benefits while minimizing the potential risks for adverse effects. For this reason, the American Society of Parenteral and Enteral Nutrition (ASPEN) keeps on providing us of guidelines and recommendations about PN safety (7). One of the changes that surely improved HPN safety has been the introduction in the clinical practice of multichambered bags (the so-called ‘all-in-one bags’). The ASPEN guidelines suggest their use as an available option to best meet patient needs (7).
PN complications
The first rule to avoid PN complications is to prevent them. Complications from PN can be divided into four categories: (I) metabolic; (II) infectious; (III) mechanical; and (IV) psychological. Usually, the metabolic complications can occur acutely, such as hyperglycemia, electrolyte disturbances, and altered hydration status. These complications are rare and easy to manage. Differently, metabolic complications such as metabolic bone disease and liver dysfunctions [the so called PN associated liver dysfunction (PNALD)] may be associated with long-term PN use (i.e., years). However, this is not the case for cancer patients on HPN that usually have a median survival ranging between 6 and 12 months (8,9).
Refeeding syndrome (RS) describes the biochemical changes (electrolyte abnormalities), clinical manifestations (fluid retention), and potential complications (cardiorespiratory dysfunction) that can occur as a consequence of feeding a severe malnourished person. Indeed, this complication may occur only in extreme cases, such as in cancer patients with a body mass index less of 14 or a starvation longer than 15 days. In these patients, RS can be prevented by a stepwise and tailored refeeding protocol as well as providing optimal management and monitoring. A prophylactic supplement of phosphate should be always prescribed as well as serum phosphate levels should be closely monitored in patients at risk of the RS. Also, a restricted policy regarding sodium and IV fluids should be adopted in order to maintain zero balance. Finally, consider thiamine supplements (to the level of 100–300 mg/day) during the first 3 days in order to prevent neurological side effects associated with glucose delivery from PN (10).
In the past, the use of high doses of glucose resulted in hyperglycemia, hypertriglyceridemia, and hepatic steatosis. Nowadays, glucose-related abnormalities can be prevented to a large extent by choosing parenteral mixtures with a reduced glucose content. For the few cancer patients on HPN that need to continue insulin therapy (e.g., patients with diabetes, steroid therapy or pancreatic cancer), it is important they to be educated about glycemic control and be provided with a glucometer. Lipid-related abnormalities occur very rarely in cancer patients on HPN, usually related to liver dysfunctions (i.e., cholestasis) due to the progression of cancer disease in the liver. When a triglyceride level greater than 5 mmol/dL (or >400 mg/dL) is reached, the fat content may be reduced (i.e., opening the bag lipid compartment from 1 to 4 times per week) according to the triglyceride level.
An interesting question is: which is the most feared and relevant complication of PN in cancer patient? The answer is catheter-related bloodstream infection (CRBSI), but this is just catheter-related and not PN-induced complication. Also, mechanical complications (i.e., catheter dislocation, lumen occlusion, rupture of external tract, and venous thrombosis), as infectious complications, are catheter-related complications (CRCs) and not PN-induced complications. In cancer patients is particularly important the prevention of catheter-related central venous thrombosis due to a higher risk of deep vein thrombosis in these patients. Thrombosis is avoided by the use of appropriate insertion techniques including: (I) the ultrasound guidance at insertion; (II) the choice of a catheter with the smallest caliber possible; and (III) the position of the catheter tip at or near the atriocaval junction (11).
In recent years, several technological novelties have considerably improved the safety of central venous access devices (VADs) in cancer patients (i.e., peripheral insertion of VAD, ultrasound-guided venipuncture, novel materials, and sutureless devices for catheter securement), whereas new policies have successfully decreased the overall risk of complications (well-defined ‘bundles’ of evidence-based interventions, strict policies on hand washing, proper skin antisepsis, training of healthcare professionals, etc.) (12).
Finally, which is the psychological impact of HPN on patient’s quality of life (QoL)? According to the literature, HPN improve QoL in advanced cancer patients (13-15). An important factor that markedly reduces psychological impact of HPN is the infusion delivery during the nighttime. Like this, HPN has minimal impact on a patient’s daily activities.
The second rule to avoid PN complications is monitoring cancer patients on HPN. Every healthcare provider involved in the care of cancer patient should be prepared to recognize signs of CRCs and early intervene for treating them. In our management process, the occurrence of CRCs is closely monitored by the physician responsible for HPN through regularly scheduled and structured telephone interviews (at least every 15 days), in-hospital medical examinations (at least every month), and home visits by specifically trained nurses (initially every day for 2–3 weeks and at least every 7 days thereafter). If accurately managed, HPN can be safely provided for most cancer patients, even in an advanced stage, without expecting a relevant incidence of CRCs (16).
Enteral nutrition (EN)
EN is the preferred method of nutritional support when the GI tract is functional and the cancer patient is unable to have an adequate oral intake of nutrients to meet his/her nutritional requirements. The guidelines (3,4,17) recommend that EN may be done using nasogastric tube (NGT) or percutaneous endoscopic gastrostomy (PEG) in radiotherapy—induced severe mucositis or in head-neck/thoracic cancers with obstructive tumor masses. Long-term home EN may be provided, usually through a PEG. About 10% of head and neck cancer patients require permanent EN (8). The enteral route is efficient and cost-effective, however it is not always as easy as it looks.
EN complications
Also EN may cause complications that can be divided into three categories: (I) GI; (II) mechanical; and (III) metabolic. Early satiety, nausea, and vomiting occur in approximately 20% of patients receiving EN due to several causes—usually, the pathogenesis is multifactorial in cancer patient—but delayed gastric emptying is the most common cause. If delayed gastric emptying is suspected, consider the following strategies: (I) reducing the rate of infusion; (II) reducing opioid medications—if it is possible; (III) switching to a low-fat enteral formula; (IV) administering the enteral formula at room temperature; and (V) finally, administering prokinetic and/or antiemetic medications. If the patient present abdominal distension, check gastric residuals before the next bolus feeding or every 4 hours for continuous feeding. During EN administration, diarrhea is common, occurring in 2–63% of patients; obviously, the incidence is depending on how diarrhea it is defined (i.e., as having 3 or more loose or liquid stools per day, or as having more stools than is normal for that person). Constipation is less common and usually is more probably due to the disease (i.e., peritoneal carcinomatosis and/or intra-abdominal recurrences) than the EN formula.
Pulmonary aspiration is the most feared and relevant complication of EN and can be life-threatening. The incidence of clinically significant aspiration pneumonia is rare. However, aspiration of small amounts of EN formula may not cause immediate symptoms, but the appearance of fever in a cancer patient in EN requests to exclude a aspiration pneumonia. As for PN, mechanical complications are tube-related and may arise during the placement of the EN access device into the GI tract or later from its presence. The more common complications are tube malposition and clogging. Several studies compared the rate of tube-related complications in case of NGT or PEG. There was not sufficient evidence to determine between NGT and PEG the optimal method of enteral feeding for patients with head and neck cancer receiving radiotherapy and/or chemoradiotherapy (18). However, head and neck cancer patients with NGT feeding were more likely to experience tube dislodgement, while NGT caused less incidence of dysphagia than PEG (19). However, cancer is identified as a significant risk factor of PEG-related infection (20).
Metabolic complications of EN are similar to those that occur during PN. RS may occur also with EN. As for PN, careful monitoring can minimize or prevent these complications.
Enteral versus parenteral nutrition
Initially, PN was considered to be the standard of care when a patient needs AN. Without any doubt the introduction of PN in the clinical practice in the late 1960s significantly helped many surgical and critically ill patients to recover from previously life-threatening clinical conditions. However, the widespread use of this treatment, in the form of ‘hyperalimentation’ (i.e., hypercaloric PN)—also called overfeeding—in all patients even with uncertain indications generated doubts and mistrust and consequently increased the role of EN (21).
This is so true that, at the beginning of this millennium, Heyland wrote in an editorial there are limited data demonstrating that PN positively impacts clinically-relevant end points in critically ill patients. Moreover, Heyland asserted that studies comparing EN with PN suggest that EN is associated with reduced infectious complications while PN is associated with increased morbidity and mortality in some subgroups of critically ill patients (22). In contrast, Jeejeebhoy, another master of nutrition, in the same years had a completely opposing opinion regarding PN and wrote that the dangers of PN-induced complications have been exaggerated. Further, Jeejeebhoy clearly and concisely defined in his editorial the role of PN writing that ‘PN is an equally effective alternative to EN when a risk of malnutrition is present and EN is not tolerated or when gut failure is present’ (23).
In 2014, it was published a RCT comparing early NE with early PN in 2,400 critically ill patients. The results were: (I) there were no significant differences between the parenteral and the enteral group in the mean number of treated infectious complications, in rates of 14 other secondary outcomes, or in rates of adverse events; (II) caloric intake was similar in the two groups, with the target intake not achieved in most patients. The conclusions of the authors were: (I) no significant difference in 30-day mortality associated with the route of delivery of early nutritional support in critically ill adults; (II) early nutritional support through the parenteral route, as it is typically administered, is neither more harmful nor more beneficial than such support through the enteral route (24).
In the late 1980s emerging evidence from animal studies supported the concept that EN promotes gut function and prevents the translocation of intestinal bacteria. Therefore, total PN was considered to be a ‘dangerous’ form of therapy (e.g., ‘more harm than good’ or ‘a poison’) and this belief resulted in EN becoming the new standard of care in AN. These studies influenced the choice of ICU physicians of route for delivering nutrients. Moreover, these conclusions were translated in the clinical practice for the nutrition support of all other patients requiring AN. However, the cancer patient is different from the ICU patient in total PN. Usually, cancer patients are not aphagic; particularly patients on anticancer treatment. Aphagic patients receiving total HPN are less than 10% in our experience while the others cancer patients in HPN have residual—but insufficient—oral food intake (usually, a median of 500 Kcal per day) (16). These non-aphagic cancer patients underwent supplemental PN (SPN). Specifically, SPN provides additional amino acids and energy to offset the protein and weight loss experienced from declining food intake. SPN at home provides a median amount of 1,000–1,250 kcal per day, from 3 to 6 times per week. Moreover, there are some advantages of SPN in comparison with total PN: low risk of overfeeding—and therefore of hyperglycemia—overhydratation, and liver dysfunction. Finally, the QoL is improved by a non-daily infusion of HPN.
In cancer patients, only a few old studies that were randomized (25-27) or prospective and controlled (27), compared short-term EN with PN. These studies reported that PN was more effective than EN in achieving weight gain (27), even though this gain may be due to an increase of fat mass or water. Moreover, these studies showed that PN was able to preserve a better nitrogen balance and plasma amino acid level (25-27).
Actually, the choice between EN and PN depends on the disease site. A recent study in 1903 French cancer patients showed that the percentage of patients receiving EN (13.8) was not so far from that of patients receiving PN (9.6) (28); particularly, patients with pancreatic, colorectal, gynecological, and hematologic tumors receiving PN were 2–4-fold more than those receiving EN.
The most common reason because a cancer patient may need nutrition support is that he/she very often experiences food intake problems due to negative side effects of the anticancer treatments (surgery, chemotherapy, and radiation therapy). If patients develop GI toxicity from neoadjuvant therapy (i.e., radiation enteritis or chemotherapy/radiation-induced diarrhea), short-term PN is usually better tolerated and more efficient than EN to restore the intestinal function and prevent nutritional deterioration (2). Actually, in cancer patients the GI tract is not always able to tolerate the infusion of the amount of EN formula to meet patients’ nutritional requirements due to peritoneal carcinomatosis and/or intra-abdominal recurrences. Moreover, many patients do not tolerate NGT or refuse the placement of PEG and/or jejunostomy. Besides, Orrevall et al. showed that nausea, vomiting, and GI obstructions were the most common indications for PN in palliative patients (29). Finally, PN should be initiate if adequate EN is not possible in patients with severe radiotherapy-induced mucositis or enteritis and head-neck/esophageal obstructive cancer masses (4).
In the past, many physicians were concerned to start HPN because of the risks potentially associated with the placement and management of central VADs. Currently, this consideration should not still have influence on the decision to feed a cancer patient when PN is clinically indicated. Besides, nowadays almost all cancer patients have a central VAD for a safe administration of chemotherapy.
A criticism directed at PN is to be over twice the cost of EN. Indeed, evidence clearly demonstrated that both EN and PN are relatively cheap adjuvant therapies—helpful to enhance effectiveness of anticancer therapies—especially if compared to other treatments (30). On the contrary, a prolonged in-hospital length of stay is dramatically more expensive than HPN.
Finally, the patient perception of the comfort of the feeding method should be the most important determinant in the choice of the route for delivering AN. Scolapio et al. (31) reported that when given a choice between PN and EN, 91% cancer patients preferred IV feeding.
Artificial nutrition in advanced cancer patients
The role of AN in advanced incurable cancer patients is without doubt the most controversial topic in debate today. There are pros and cons regarding the use of AN in this oncology population. First of all, we have to distinguish advanced cancer patients between incurable and end-of-life patients, because it is crucial to be sure that the debate refers to the same kind of patients. Worldwide accepted guidelines (2-4) recommend to feed malnourished incurable cancer patients who are still in active treatment with adequate nutrition support in order to enhance compliance with anticancer treatments and control some adverse effects of anticancer therapies (32). Actually, muscle loss strongly predicts the development of chemotherapy-related dose-limiting toxicity (33).
Is there an evidence according to the results of a RCT? Unfortunately no RCT was carried out, because any prospectively controlled evidence of potential benefit is denied due to the unethical nature of longer-term studies having a non-AN control arm including aphagic patients or with markedly insufficient oral food intake (2).
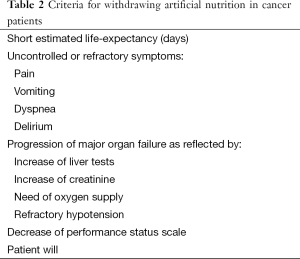
Nowadays, there is an increasing number of cancer patients who survive in a chronic condition of reduced oral food intake. But, the key question is: is there the indication for HAN in patients with no further treatments? (34). The European guidelines state that stopping anticancer treatments is not a contraindication for HAN (2). The rationale is that some advanced cancer patients, especially those with GI tract tumors may die from progressive starvation but not because the tumor (8). Incurable cancer patients should receive nutritional interventions if their expected benefit outweighs the potential harm and, obviously, the patient wants this therapy (4). However, HAN should not be prescribed to all patients with incurable cancers. The major challenge is how to identify patients with a performance status and tumor stage/spread which allow them to survive more than 3 months (9). In these patients, is really important to discuss the role of nutrition support in this phase of their disease. In the Table 1 are shown our criteria for withholding AN in cancer patients. Finally, in patients who are imminently dying artificial hydration and nutrition are unlikely to provide any benefit. However, providing AN or artificial hydration to cancer patients who are in the last week of life is a frequent practice (35). In the Table 2 are shown our criteria for withdrawing AN in cancer patients.

Full table
Full table
In the past, it was a common experience that the vast majority of cancer patients referred to the nutrition team for nutritional evaluation had an evident cachexia. According with the classification of cancer cachexia (36), refractory cachexia is characterized by a low performance status [Eastern Cooperative Oncology Group (ECOG) 3 or 4] and an estimated life expectancy of less than 3 months. In this phase of disease trajectory, the cancer patient is not responsive to anticancer treatment as well to AN aiming to reverse cachexia. In fact, an opportunity for nutritional interventions to stop or reverse cachexia exists when the expected survival is more than 3 months (37). Therefore, therapeutic interventions should focus on alleviating the consequences and complications of cachexia, i.e., symptom palliation (appetite stimulation, management of nausea or eating-related distress of patients and caregivers).
Conclusions
AN is essential to meet the nutritional needs of cancer patients at risk of undernutrition as the latter can lead to a poorer prognosis for these patients. There is debate over which method of AN provides the most benefit to the cancer patient for outcomes such as nutritional benefit and QoL, as well as avoiding delays in anticancer treatments. However, due to the small number of comparative studies available, we have no evidence-based data able to definitively indicate the optimal method for delivering AN in cancer patients.
In summary, EN and PN have to be considered equally effective in maintaining or improving nutritional status in cancer patients (8). Besides, this review strongly supports the recommendation that a baseline nutritional assessment should be carried out by a healthcare professional expert in AN for all cancer patients at the time of diagnosis or anticancer treatment plan, taking the nutritional status, estimated duration of AN, AN-related potential benefits and possible complications into consideration on an individual basis. Moreover, the patient symptoms, performance status, estimated life expectancy, and mainly, will or preferences have to be evaluated and incorporated into the nutrition support plan before the definitive choice of the route for delivering nutrients is decided. Finally, applying a decision-making process tailored to patient needs—regardless of whether receiving or not anticancer treatment—allows to choose reasonably the optimal nutritional support strategy.
Acknowledgements
The Author is in debt with the following Colleagues for their intellectual contribution in his expertise in the field of artificial nutrition in cancer patients: Federico Bozzetti, Augusta Palmo, and Mauro Pittiruti.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Chow R, Bruera E, Chiu L, et al. Enteral and parenteral nutrition in cancer patients: a systematic review and meta-analysis. Ann Palliat Med 2016;5:30-41.
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- August DA, Huhmann MB; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. [PubMed]
- Arends J. ESPEN Guidelines: nutrition support in cancer. (Accessed on Dec. 30, 2015). Available online: http://www.espen.org/presfile/Arends_J_2014.pdf
- Staun M, Pironi L, Bozzetti F, et al. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr 2009;28:467-79. [PubMed]
- Hvas CL, Farrer K, Donaldson E, et al. Quality and safety impact on the provision of parenteral nutrition through introduction of a nutrition support team. Eur J Clin Nutr 2014;68:1294-9. [PubMed]
- Boullata JI, Gilbert K, Sacks G, et al. A.S.P.E.N. clinical guidelines: parenteral nutrition ordering, order review, compounding, labeling, and dispensing. JPEN J Parenter Enteral Nutr 2014;38:334-77. [PubMed]
- Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol 2013;87:172-200. [PubMed]
- Bozzetti F, Cotogni P, Lo Vullo S, et al. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann Oncol 2015;26:2335-40. [PubMed]
- Khan LU, Ahmed J, Khan S, et al. Refeeding syndrome: a literature review. Gastroenterol Res Pract 2011;2011.
- Pittiruti M, Hamilton H, Biffi R, et al. ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr 2009;28:365-77. [PubMed]
- Cotogni P, Barbero C, Garrino C, et al. Peripherally inserted central catheters in non-hospitalized cancer patients: 5-year results of a prospective study. Support Care Cancer 2015;23:403-9. [PubMed]
- Bozzetti F, Cozzaglio L, Biganzoli E, et al. Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clin Nutr 2002;21:281-8. [PubMed]
- Vashi PG, Dahlk S, Popiel B, et al. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014;14:593. [PubMed]
- Culine S, Chambrier C, Tadmouri A, et al. Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: a French observational multicentre study. Support Care Cancer 2014;22:1867-74. [PubMed]
- Cotogni P, Pittiruti M, Barbero C, et al. Catheter-related complications in cancer patients on home parenteral nutrition: a prospective study of over 51,000 catheter days. JPEN J Parenter Enteral Nutr 2013;37:375-83. [PubMed]
- Arends J, Bodoky G, Bozzetti F, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr 2006;25:245-59. [PubMed]
- Nugent B, Lewis S, O'Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev 2013;1:CD007904. [PubMed]
- Wang J, Liu M, Liu C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Radiat Res 2014;55:559-67. [PubMed]
- Richter-Schrag HJ, Richter S, Ruthmann O, et al. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol 2011;25:201-6. [PubMed]
- Klek S. Immunonutrition in cancer patients. Nutrition 2011;27:144-5. [PubMed]
- Heyland DK. Parenteral nutrition in the critically-ill patient: more harm than good? Proc Nutr Soc 2000;59:457-66. [PubMed]
- Jeejeebhoy KN. Total parenteral nutrition: potion or poison? Am J Clin Nutr 2001;74:160-3. [PubMed]
- Harvey SE, Parrott F, Harrison DA, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673-84. [PubMed]
- Lim ST, Choa RG, Lam KH, et al. Total parenteral nutrition versus gastrostomy in the preoperative preparation of patients with carcinoma of the oesophagus. Br J Surg 1981;68:69-72. [PubMed]
- Pearlstone DB, Lee JI, Alexander RH, et al. Effect of enteral and parenteral nutrition on amino acid levels in cancer patients. JPEN J Parenter Enteral Nutr 1995;19:204-8. [PubMed]
- Burt ME, Stein TP, Brennan MF. A controlled, randomized trial evaluating the effects of enteral and parenteral nutrition on protein metabolism in cancer-bearing man. J Surg Res 1983;34:303-14. [PubMed]
- Hébuterne X, Lemarié E, Michallet M, et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196-204. [PubMed]
- Orrevall Y, Tishelman C, Permert J, et al. A national observational study of the prevalence and use of enteral tube feeding, parenteral nutrition and intravenous glucose in cancer patients enrolled in specialized palliative care. Nutrients 2013;5:267-82. [PubMed]
- Laviano A, Fearon KC. The oncology wall: Could Ali Baba have got to the nutrition treasure without using the correct words? Clin Nutr 2013;32:6-7. [PubMed]
- Scolapio JS, Picco MF, Tarrosa VB. Enteral versus parenteral nutrition: the patient's preference. JPEN J Parenter Enteral Nutr 2002;26:248-50. [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007;13:3264-8. [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920-6. [PubMed]
- Bozzetti F. The patient with incurable aphagic cancer: to feed or not to feed? Nutrition 2001;17:676-7. [PubMed]
- Raijmakers NJ, van Zuylen L, Costantini M, et al. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literature review of practices and effects. Ann Oncol 2011;22:1478-86. [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [PubMed]
- Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012-9. [PubMed]


