Comparison of the EORTC STO-22 and the FACT-Ga quality of life questionnaires for patients with gastric cancer
Introduction
Gastric cancer is the third leading cause of cancer mortality (1). It remains a health concern by accounting for 6.8% of new cancer cases globally and 8.8% of total cancer deaths worldwide (1,2). Recently, there has been an increase in the proportion of advanced (stage IV) gastric cancer which now comprises over 40% of total cases (3). In North America, approximately 65% of gastric cancers are identified at an advanced stage (4).
The prognosis for gastric cancer is poor, with a 5-year survival rate ranging from 22% to 27% in Western countries (5,6). Irrespective of disease severity, patients may face significant limitations to both their physical and social functioning (7). Treatment options include surgical resection, radiotherapy or chemotherapy, all of which have the potential to cause significant treatment-related adverse events (7-9). Given the poor prognosis and debilitating course of disease, interventions for advanced gastric cancer are typically palliative in nature and thus survival may not be the only significant endpoint.
Recently, quality of life (QOL) has emerged as an increasingly important outcome to be considered alongside traditional oncologic outcomes such as survival and locoregional control (10-13). QOL is a subjective, multi-dimensional concept encompassing physical, psychological, and social issues (8,10). A growing body of literature has recognized QOL as an important outcome used to complement traditional endpoints such as disease-free and overall survival (10-13). Understanding and assessing QOL is critical to the holistic management of patients and may assist clinicians in determining an optimal treatment regimen (14). QOL is typically assessed through self-reported questionnaires completed by the patient or via proxy.
Two widely used QOL assessment questionnaires for patients with any type of cancer are the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) and the Functional Assessment of Cancer Therapy-General (FACT-G). The EORTC QLQ-C30 is a 30-item questionnaire that incorporates nine domains of QOL including physical, cognitive, emotional, role and social functional scales, as well as symptom scales for pain, fatigue, nausea/vomiting and overall health of the patient (12). In contrast, the FACT-G is a 28-item questionnaire that addresses four primary QOL domains: physical well-being (PWB), social/family well-being (SWB), emotional well-being (EWB), and functional well-being (FWB) (15).
The EORTC Quality of Life Questionnaire-Stomach (QLQ-STO22) and FACT-Gastric (FACT-Ga) are gastric cancer-specific modules which have been developed to be used in combination with their respective core questionnaires. The QLQ-STO22 is a 22-item instrument that is used alongside the 30-item QLQ-C30 core questionnaire, resulting in a total of 52 items. In comparison, the FACT-Ga is a 19-item module used to complement the 28-item FACT-G core questionnaire for a total of 47 items (9,16).
While other instruments exist for the assessment of QOL among gastric cancer patients, the EORTC QLQ-STO22 and FACT-Ga have been applied in clinical trials worldwide. Currently, no review articles have directly compared the EORTC QLQ-STO22 and the FACT-Ga (9,17-23). As such, the purpose of this systematic review is to compare and contrast the development, characteristics, and reliability/validity of the English versions of these two questionnaires.
Materials and methods
A literature search was conducted using Ovid MEDLINE and OLDMEDLINE (inception to April 2015 week 3), Cochrane Central Register of Controlled Trial (inception to March 2015) and Ovid EMBASE and EMBASE Classic (inception to 2015 Week 17) to identify studies that discussed the development, characteristics, validity, and reliability of the EORTC QLQ-STO22 or the FACT-Ga. Search terms “stomach neoplasm”, “stomach”, “gastric”, “cancer”, “neoplasm”, “tumor” and “tumour” were combined with “EORTC”, “FACT”, “questionnaire”, “survey”, instrument”, “assess”, “evaluate”, “QLQ” and “FACT-Ga” to elicit relevant literature. The bibliographies of included studies were also searched for relevant articles.
To assess individual articles for eligibility, two reviewers (A.W., T.F.) independently screened all identified studies by titles and abstracts, and then by full-text versions. Studies were included if they discussed at least one of the following for either QOL tool: development, characteristics, and/or validity. Non-English studies and articles reporting on previously documented data were excluded. We extracted information related to the development process, characteristics, intended use, and validation process of the two questionnaires.
Results
The literature search identified a total of 288 articles (Figure 1). In order from most to least common, the reasons for exclusion were: duplicates [78], studies of other QOL questionnaires [72], studies providing insufficient information [64], non-gastric cancer studies [47], and non-original studies [16]. Inter-rater agreement for study inclusion was excellent (κ=0.95). In total, 11 studies were selected for inclusion. From these, six articles discussed the development, characteristics, and/or validity for the EORTC QLQ-STO22, while five articles provided relevant information on the FACT-Ga.
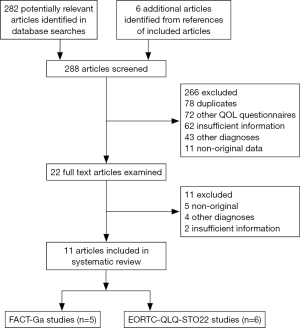
Development
Vickery et al. (13) first characterized the development of the EORTC QLQ-STO22. The process was conducted according to the EORTC QOL Group guidelines in four distinct phases. In phase I, an extensive literature review was carried out and produced a list of 42 potentially relevant QOL issues addressing common symptoms of gastric cancer and treatment side effects. Twenty-four health care professionals (gastrointestinal surgeons, oncologists, gastroenterologists, palliative care consultants, general practitioners and specialist nurses) and 58 patients from four European countries were interviewed to rate the relevance of each item and to suggest additional issues for inclusion. A total of 20 issues were identified at the conclusion of phase I. Phase II of development involved rewording all of the QOL issues into questionnaire items. A provisional version of the module containing 24 items was produced and translated into French, German and Spanish according to the translation guidelines by the EORTC QOL Group (24). During phase III, pre-testing was performed on 115 patients from France, Germany, Spain and the United Kingdom who completed both the EORTC QLQ-C30 and the provisional version of the module. Following pre-testing, several items were added, modified or deleted. Phase III led to the development of the 22-item EORTC QLQ-STO22, which was then translated into nine European languages for international field-testing. Phase IV involved psychometric testing of the module’s reliability, validity and sensitivity to change. The module was then field tested with 219 patients from 14 institutions in eight different countries (17). Patients completed the questionnaire at three points in time during field testing: baseline, 4 weeks, and 3 months. As a result, item 34 in the dysphagia scale addressing discomfort during eating was included under the pain scale and item 45 addressing taste problems was presented as a single item in the final module. The final version of the module contained a total of 22 items.
Eremenco et al. (16) documented the development of the FACT-Ga, which was designed concurrently in North America and Asia to ensure that it demonstrated cross-cultural validity. This gastric cancer-specific module was produced according to a standard development process by the Functional Assessment of Chronic Illness Therapy (FACIT) organization. A provisional module of 65 items specific to gastric cancer was first generated from interviews with 17 patients and 12 healthcare professionals. A rigorous qualitative data review reduced the module to 36 items, and a provisional module was constructed. In the first stage of psychometric testing, the validity of the provisional module was tested on 30 patients (20 Japanese and 10 Canadian), who evaluated the relevance and importance of each item. The content of each item was then modified based on feedback from participants and 13 experts from the US, Canada, and Japan. This version of the FACT-Ga performed well in this initial validation with high reliability in both patient groups. Seven items were removed from the provisional questionnaire due to redundancy, resulting in a 19-item module. After further refinements of the item scales, the module was translated into Japanese using the FACIT translation methodology. The English and Japanese versions of the FACT-Ga were validated and shown to be suitable for use in English and Japanese research studies and clinical trials. In the second stage of psychometric testing (9), 62 patients with gastric adenocarcinoma completed the FACT-Ga at three time points: baseline, 2 weeks, and 3 months. The tests demonstrated an acceptable level of validity, reliability as well as sensitivity to change in QOL assessed by the FACT-Ga.
Characteristics
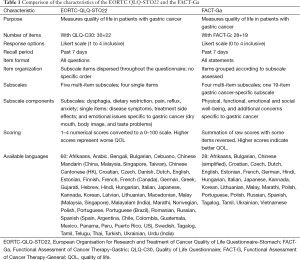
A comparison of the characteristics of the EORTC QLQ-STO22 and the FACT-Ga is shown on Table 1. Both questionnaires are used to evaluate QOL in gastric cancer patients. The EORTC QLQ-STO22 is administered alongside the 30-item EORTC QLQ-C30 core questionnaire, which includes a global health status assessment, five multi-item functional subscales, and several single or multiple-item symptom subscales. Items of the QLQ-C30 are assessed on a 4- or 7-level Likert scale with options ranging from 1= “very poor” to 7= “excellent” for global health status items and, 1= “not at all” to 4= “very much” for all other items (12,19). The gastric cancer-specific QLQ-STO22 includes a total of 22 items, comprising five multi-item and four single-item subscales. The multi-item subscales include questions about dysphagia (4 items), dietary restriction (5 items), pain (3 items), upper gastro-esophageal symptoms such as reflux (3 items), and emotional problems such as anxiety (3 items). The single-item subscales include questions related to four gastric cancer-specific symptoms: dry mouth, body image, hair loss, and problems with taste. Subscale items are dispersed throughout the questionnaire with no specific order. The EORTC QLQ-STO22 items are presented as questions. Patients are asked to recall their QOL issues over the past week, and items are assessed on a 4-level numerical scale with 1= “not at all”, 2= “a little”, 3= “quite a bit”, and 4= “very much”. Scores are linearly converted and summated into a scaled score from 0 to 100, with a higher score representing a worse QOL (13,17).
Full table
In contrast, the FACT-G is a 28-item core questionnaire consisting of four subscales that assess PWB, EWB, SWB, and FWB (9). The FACT-Ga combines the FACT-G with a 19-item module which addresses additional gastric cancer specific concerns and symptoms. Items are grouped by their subscales. The FACT-Ga items are presented as statements instead of questions. Similar to the EORTC QLQ-STO22, patients are asked to recall their QOL issues within the past week. As well, the questionnaire is assessed on a 5-level numerical scale with 0= “not at all”, 1= “a little bit”, 2= “somewhat”, 3= “quite a bit”, and 4= “very much”. In terms of scoring methodology, the FACT-Ga scores are summed with total scores ranging from 0 to 184. Scores can also be reported for each subscale individually (e.g., a FWB score). In contrast to the QLQ-STO22, a higher score indicates a better overall QOL. Some items require score reversal to accommodate this scoring method.
The EORTC QLQ-STO22 has been translated into 60 languages while the FACT-Ga has been translated into 28 languages, as shown on Table 1 (25-27).
Validity
The validity and reliability of the EORTC QLQ-STO22 was investigated in an article by Blazeby et al. (17). An overall sample size of 219 gastric cancer patients were recruited from 14 institutions in eight countries (UK, France, Spain, Germany, Republic of Ireland, Australia, Turkey and Belgium), and divided into two groups: (I) patients selected for potentially curative treatment (group A); and (II) patients selected for palliative treatment (group B). Multi-trait scaling was used to analyze the hypothesized scale structure of the EORTC QLQ-STO22. For internal consistency testing, a Cronbach’s alpha coefficient α ≥0.70 was considered to be acceptable for group comparison. The coefficient was lowest in the reflux and anxiety scales (0.72 and 0.73 respectively) and was 0.80 for the remaining scales. Item-scale correlations were also calculated and all values surpassed the 0.40 standard set by the EORTC (range, 0.60 to 0.77), thus indicating desirable convergent validity. The results of the multi-trait scaling analysis led to two modifications: (I) item 34, addressing discomfort while eating was moved from the dysphagia scale to the pain scale; and (II) item 45, addressing taste issues was initially part of the eating restriction subscale, but was changed to a single item in the final module.
Test-retest reliability was evaluated using an intraclass correlation between perioperative and retest assessments at 3 months post-treatment. Here, the questionnaires were administered to 24 patients in group A undergoing surgery alone at UK institutions (17). Pain, eating restriction, and anxiety scales were reproducible to an acceptable standard as intraclass correlations were above 0.70. Single item interclass correlations also showed a good reliability, with correlations above 0.79. However, the dysphagia and reflux scales yielded questionable reliability with interclass correlations of 0.60 and 0.63, respectively.
Construct validity was evaluated using the correlations between the EORTC QLQ-STO22 module and the scales of the QLQ-C30 core questionnaire (17). A high degree of correlation would indicate redundancy between the two scales; conversely, low correlation would indicate that the QLQ-STO22 module adds value by assessing distinct areas of QOL. The article revealed that most scales in the QLQ-STO22 were weakly correlated to the QLQ-C30 scales. Additionally, the gastric dysphagia, eating restriction, and pain scales were found to be moderately correlated to those in the core questionnaire. Although redundant items from the QLQ-C30 were supposedly removed during the EORTC QLQ-STO22 development process, it was expected that some clinical overlap and correlation would remain. Ultimately, the scales were not modified because of the importance of assessing dysphagia, eating and pain issues in the gastric cancer module.
Finally, the authors assessed the responsiveness of the EORTC QLQ-STO22 to changes in health status over time by comparing questionnaire responses completed before and after treatment (17). The analysis showed that the reflux scale demonstrated sensitivity to changes in weight loss over time (P=0.003), and scales assessing dysphagia, pain, reflux and eating were all sensitive to changes in observer-rated dysphagia scores (P<0.01). In addition, the physical function scale of the EORTC QLQ-C30 was strongly related to changes in patients’ functional status over time as measured by the Karnofsky Performance Status (KPS) (P<0.01). Changes in QOL were also examined based on treatment group. For instance, patients reported decreased physical function and increased fatigue, diarrhea, and poor body image three months post-gastrectomy (P<0.01). After receiving palliative treatment, patients also reported diminished physical function, taste, and hair loss (P<0.01).
Similarly, the FACT-Ga has been proven to be valid and reliable. A pilot testing of the English and Japanese versions of the module were carried out on an initial sample of 30 gastric cancer patients (10 Canadians and 20 Japanese) for content refinement (9). With a threshold of acceptability of α≥0.70, both versions demonstrated a satisfactory internal consistency with Cronbach’s alpha coefficient reaching 0.93 in the English-speaking group and 0.84 in the Japanese-speaking group. In a study by Garland et al. (9), 62 patients in Canada with gastric cancer of varying stages were recruited for the second stage of validation of the FACT-Ga. Assessment was carried out at three separate points: at baseline, 2 weeks and 3 months later. The internal consistency of each subscale and the overall questionnaire were again tested and determined by calculating the Cronbach’s alpha coefficients. The FACT-Ga and each of its gastric cancer-specific subscales demonstrated a good internal consistency with α>0.70 in all cases (range, 0.81 to 0.86). However, the FACT-G and its EWB subscale yielded alpha coefficients of only 0.49 and 0.60, respectively. Test-retest reliability and stability were also demonstrated through satisfactory intraclass correlation coefficients between baseline and 2-week assessments. Specifically, intraclass correlation coefficients were 0.89 and 0.88 for the FACT-Ga and gastric cancer subscales, respectively, which is higher than the acceptable standard of 0.70. In a different study, Pelletier and colleagues studied 81 gastric cancer patients and also found high test-retest reliability, with an excellent correlation coefficient of 0.941 (28).
Construct validity was tested (9) using Pearson correlations between the FACT-Ga total/subscale scores and other well-established instruments measuring QOL [Short Form-36 Health Survey (SF-36)], depression [Beck Depression Inventory-II (BDI-II)], anxiety [State-Trait Anxiety Inventory (STAI)] and social desirability (Marlow-Crowne Social Desirability Scale). The FACT-Ga and all subscales were correlated to all eight health concepts represented by the SF-36 (r=0.623 to 0.737), with the exception of the SWB subscale, which was not strongly correlated to any of the QOL measures (r=0.050 to 0.220). In addition, the FACT-Ga was negatively correlated to measures of anxiety (r=−0.752) and depression (r=−0.563). Similar results have been reported by Pelletier et al. (28), who found a positive correlation between the FACT-Ga and the SF-36 (r=0.52 to 0.84) and a significant negative correlation with unrelated indicators such as the BDI-II and STAI (r=−0.74 and −0.57, respectively).
Criterion validity, a measure of how well a module predicts a certain disease-related outcome, was established by comparing the FACT-Ga across various stages of cancer (9). Patients with resectable disease (stage I, II, III) were compared to those with unresectable disease (stage IV). Through this analysis, the FACT-Ga and its subscales were significantly related to disease stage. Sensitivity to detecting changes in QOL was also assessed by comparing patient-reported QOL scores on the FACT-Ga with the KPS assigned by the physician. Patients with physician-reported deteriorating KPS reported a greater change in FACT-Ga, PWB, FWB, and gastric symptoms compared to patients with unchanged or improved KPS.
Finally, minimally important difference (MID) scores were calculated by assessing the strength of relation between the change of QOL and change in module scores (9). The r2 value for the FACT-G, FACT-Ga total, and the gastric cancer subscale were 0.37, 0.41, and 0.44, respectively. The results demonstrated that the FACT-G and total FACT-Ga were good predictors of change in QOL among patients with gastric cancer.
Discussion
Currently, an array of questionnaires exists for the measurement of QOL among patients with gastric cancer (11). This can be overwhelming for researchers and clinicians who wish to select a QOL instrument with effective psychometric properties that is pertinent to their specific patient population. The present systematic review aims to compare the development, characteristics, and validity of two widely used QOL questionnaires for gastric cancer, the EORTC QLQ-STO22 and FACT-Ga.
While both questionnaires assess QOL among patients with gastric cancer, they have a different proportion of items dedicated to various domains of QOL. For instance, the EORTC QLQ-C30 and QLQ-STO22 somewhat emphasizes the functional and physical concerns of patients (39 out of 52 items). As well, with regards to dietary QOL issues, the QLQ-STO22 explores detailed patient concerns concerning solid, liquidized/soft, and liquid food (three items). Furthermore, the EORTC QLQ-STO22 is comprehensive in its assessment of stomach pain, with questions concerning associated symptoms such as bloating or heartburn, provoking factors such as discomfort on eating, and a total of four items dedicated to assessing pain. The FACT-Ga includes only one item on dietary issues and two items dedicated to stomach pain. In contrast, the FACT-Ga focuses somewhat more heavily on the emotional and social issues of patients (18 out of 47 items), with two subscales dedicated to assessing specific questions on SWB and EWB (e.g., “I am satisfied with how I am coping with my illness”). In comparison, the QLQ-STO22 assesses a patient’s emotional and social well-being with more general questioning (e.g., “have you worried about your health in the future?”). Therefore, the selection of a questionnaire depends on the specific patient characteristics and needs.
In one validation study of the EORTC QLQ-STO22, the majority of participants (82%) completed the QLQ-C30 and QLQ-STO22 in less than 15 minutes and 53% of them did not require any assistance (17). Among the group of patients that needed help, the assistance was minimal and mostly involved clarifying the interpretation of items. Most participants commented that the questions were clear (89%) and that they did not find any items upsetting (96%). While there was no comparable information available for the FACT-Ga, this questionnaire has slightly fewer items compared to the QLQ-C30 and QLQ-STO22 (47 vs. 52 items).
International field testing of the EORTC QLQ-STO22 by Blazeby et al. (17) demonstrated good sensitivity to changes in health status, including changes in weight loss, dysphagia, and KPS score. This study also reported that the module had good reliability, as all the subscales achieved a Cronbach’s alpha coefficient criteria greater than 0.70. Furthermore, there are other studies examining the validity and reliability of the translated Chinese Mandarin, Arabic, Spanish and Japanese versions of the EORTC QLQ-STO22 (19-22). One validation study of the Chinese Mandarin version reported a good reliability for all multi-item subscales (Cronbach’s alpha coefficient 0.70–0.94) except for the cognitive function subscale of the QLQ-C30 (0.30) and eating restriction of the QLQ-STO22 (0.67) (19). Similar results have been reported for the Arabic (22), Japanese (20), and Spanish (21) versions, demonstrating good cross-cultural applicability of this module. One exception was the finding of low internal consistency for the dietary restriction subscale, which was detected in both the Chinese Mandarin and Arabic validation studies (19,22). Nevertheless, the EORTC QLQ-STO22 has been developed and tested internationally among a large patient population across a wide range of treatment modalities. As a result, the EORTC QLQ-STO22 represents a meaningful assessment tool in detecting QOL changes in a diverse gastric cancer patient population.
Pilot testing and field testing of the FACT-Ga also showed satisfactory internal consistency, with the exception of the total FACT-G core questionnaire and EWB subscale which had coefficients of 0.49 and 0.60, respectively (9). Garland et al. (9) suggested that the low internal consistency of the FACT-G core questionnaire may be an artifact of the sample since previous validation reports have demonstrated higher Cronbach’s alpha values (15,29). Some authors have commented specifically on the low reliability of the EWB subscale, suggesting that this may be due to the low item-to-scale correlation in one of the items of the EWB subscale (9,23). For instance, after excluding item GE2 (“I am satisfied with how I am coping with my illness”) of the EWB subscale (item-to-scale correlation, r=0.08), it had an improved Cronbach’s alpha value of 0.72 (23). The FACT-Ga also demonstrated criterion validity with a strong sensitivity to changes in disease stage and functional status as measured by the KPS. One study found that patients with physician-reported deterioration of KPS experienced a greater change in FACT-Ga score compared to those with an unchanged or improved KPS (9). This suggests that the FACT-Ga may have an advantage in detecting deterioration in functioning as opposed to improvement. Given the poor prognosis and disease progression associated with gastric cancer, this finding supports the use of the FACT-Ga among this particular cancer population.
Two studies demonstrated the construct validity of the FACT-Ga module, finding good correlation between its domains and corresponding domains in a different QOL assessment tool termed the European Quality of Life-5 Dimensions (EQ-5D) (9,28). The SWB subscale was the only exception to show the lowest cross-instruction correlation. In addition, the FACT-Ga was shown to have good temporal stability with excellent test-retest reliability (9,28). The retest assessment was not confirmed and performed on other versions of the FACT-Ga.
Recently, the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45) was developed by the Japanese Postgastrectomy Syndrome Working Party (JPSWP) for more comprehensive evaluation and surveillance of QOL among patients who underwent gastrectomy for gastric cancer (30-32). This 45-item questionnaire contains items from the 8-Item Short-Form Health Survey (SF-8), the Gastrointestinal Symptom Rating Scale, as well as items selected by gastric surgeons in the JPSWP. The PGSAS-45 is exclusively used and validated for patients with pathologically confirmed stage I gastric cancer that has been cured through radical surgery but were suffering from post-gastrectomy syndrome. Patients with recurrence or active metastatic disease, or those treated with chemotherapy were excluded from these validation studies. Compared to the EORTC STO-22 and FACT-Ga, the PGSAS-45 contains items that are more specifically targeted to post-gastrectomy patients such as questions related to dumping syndrome which may be overlooked in the other two questionnaires (30,32). However, these items may not be necessary in the EORTC STO-22 and the FACT-Ga in assessing the QOL of patients with gastric cancer in general. Further studies are needed to validate the use of the PGSAS-45 in a broader patient population and examine its correlation with traditional QOL scales such as the EORTC STO-22 and the FACT-Ga.
While rigorous in its methods, the present study is subject to limitations. The review is limited by the relatively small number of studies examining the characteristics, development, and reliability of the EORTC QLQ-STO22 and the FACT-Ga. For instance, only one study for the QLQ-STO22 (17) and two for the FACT-Ga (9,28) described a clinical study assessing the validity and reliability of the English-versions. Furthermore, some of the included studies were based on relatively small sample sizes.
In conclusion, the EORTC QLQ-STO22 and FACT-Ga are internationally validated tools used to assess QOL in patients with gastric cancer. The QLQ-C30 and QLQ-STO22 together have a greater proportion of items dedicated to functional and physical concerns, while the FACT-Ga emphasizes social and emotional issues to a somewhat greater degree. Despite differences in their development, question content and format, and QOL domain(s) of focus, both questionnaires have shown to be effective at detecting and assessing patients’ concerns and symptoms. Ultimately, each instrument is associated with unique strengths and limitations, and no one instrument is superior to another. Careful selection of an optimal questionnaire should be based on the specific patient characteristics and goals of the study.
Acknowledgements
We thank the generous support of the Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: A Bottomley is an EORTC member and an author of various EORTC tools, including the EORTC QLQ-STO22. He also coordinated the phase IV validation study of the EORTC QLQ-STO22 alongside the EORTC. D Cella is President of FACIT.org, which licenses commercial use of the FACT-Ga in languages other than English. The other authors have no conflicts of interest to declare.
References
- Ferlay J, Ervik M, Dikshit R, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon France; 2013. Available online: http://globocan.iarc.fr
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133-45. [PubMed]
- Dassen AE, Lemmens VE, van de Poll-Franse LV, et al. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer 2010;46:1101-10. [PubMed]
- Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer 2000;88:921-32.
- Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer 2009;45:931-91. [PubMed]
- Bunt AM, Hermans J, Smit VT, et al. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol 1995;13:19-25. [PubMed]
- Kahlke V, Bestmann B, Schmid A, et al. Palliation of metastatic gastric cancer: impact of preoperative symptoms and the type of operation on survival and quality of life. World J Surg 2004;28:369-75. [PubMed]
- Conroy T, Marchal F, Blazeby JM. Quality of life in patients with oesophageal and gastric cancer: an overview. Oncology 2006;70:391-402. [PubMed]
- Garland SN, Pelletier G, Lawe A, et al. Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument. Cancer 2011;117:1302-12. [PubMed]
- Blazeby JM, Vickery CW. Quality of life in patients with cancers of the upper gastrointestinal tract. Expert Rev Anticancer Ther 2001;1:269-76. [PubMed]
- Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol 2005;11:3189-96. [PubMed]
- Pallis AG, Mouzas IA. Instruments for quality of life assessment in patients with gastrointestinal cancer. Anticancer Res 2004;24:2117-21. [PubMed]
- Vickery CW, Blazeby JM, Conroy T, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer 2001;37:966-71. [PubMed]
- Gotay CC. Assessing cancer-related quality of life across a spectrum of applications. J Natl Cancer Inst Monogr 2004.126-33. [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [PubMed]
- Eremenco SL, Cashy J, Webster K, et al. FACT-Gastric: A new international measure of QOL in gastric cancer. J Clin Oncol 2004;22:8123.
- Blazeby JM, Conroy T, Bottomley A, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer 2004;40:2260-8. [PubMed]
- Debb SM, Arnold B, Perez B, et al. Validation of the FACT-Gastric cancer quality of life questionnaire for use in Spanish-speaking countries. Psychooncology 2011;20:19-27. [PubMed]
- Huang CC, Lien HH, Sung YC, et al. Quality of life of patients with gastric cancer in Taiwan: validation and clinical application of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-STO22. Psychooncology 2007;16:945-9. [PubMed]
- Morita S, Kaptein AA, Oba K, et al. The domain structure of the EORTC QLQ-STO22 supported by Japanese validation data. Psychooncology 2008;17:474-9. [PubMed]
- Oñate-Ocaña LF, Alcántara-Pilar A, Vilar-Compte D, et al. Validation of the Mexican Spanish version of the EORTC C30 and STO22 questionnaires for the evaluation of health-related quality of life in patients with gastric cancer. Ann Surg Oncol 2009;16:88-95. [PubMed]
- Sadighi S, Montazeri A, Sedighi Z, et al. Quality of life in patients with gastric cancer: translation and psychometric evaluation of the Iranian version of EORTC QLQ-STO22. BMC Cancer 2009;9:305. [PubMed]
- Zhou HJ, So JB, Yong WP, et al. Validation of the functional assessment of cancer therapy-gastric module for the Chinese population. Health Qual Life Outcomes 2012;10:145. [PubMed]
- Dewolf L, Koller M, Velikova G, et al. EORTC Quality of Life Group Translation Procedure. Brussels: EORTC, 2009. Available online: http://groups.eortc.be/qol/sites/default/files/archives/translation_manual_2009.pdf
- Pullmer R, Linden W, Rnic K, et al. Measuring symptoms in gastrointestinal cancer: a systematic review of assessment instruments. Support Care Cancer 2014;22:2941-55. [PubMed]
- FACIT. Questionnaires. Functional Assessment of Chronic Illness Therapy. [Accessed 5 June 2015]. Available online: http://www.facit.org/FACITOrg/Questionnaires
- EORTC Quality of Life-Questionnaires. EORTC Quality of Life Department. [Accessed 5 June 2015]. Available online: http://groups.eortc.be/qol/why-do-we-need-modules
- Pelletier G, Garland G, Thomas BC, et al. Reliability and Validity of the Functional Assessment of Cancer Therapy for Stomach Cancer (FACT-Ga): a North American experience. Psycho-Oncol 2009;18:S41.
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974-86. [PubMed]
- Misawa K, Terashima M, Uenosono Y, et al. Evaluation of postgastrectomy symptoms after distal gastrectomy with Billroth-I reconstruction using the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45). Gastric Cancer 2015;18:675-81. [PubMed]
- Terashima M, Tanabe K, Yoshida M, et al. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann Surg Oncol 2014;21 Suppl 3:S370-8. [PubMed]
- Nakada K, Ikeda M, Takahashi M, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer 2015;18:147-58. [PubMed]


