Editor’s note: “Palliative Radiotherapy Column” features articles emphasizing the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L.W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles and perspectives relating to palliative radiotherapy, editorials and commentaries on recently published trials and studies.
Latest advances in the management of radiation-induced pain flare, nausea and vomiting
Introduction
Palliative radiotherapy (RT) has been well-established as an effective treatment for symptomatic bone metastases (1). In this patient population, response rates often approach 60% (2). Nevertheless, palliative RT is associated with several treatment-related side-effects including pain flare, defined as a temporary worsening in pain (3,4). In addition, RT has shown to lead to radiation-induced nausea and vomiting (RINV) (5). The management of pain flare and RINV are important objectives in supportive care. Recent developments have been investigated to add to the currently available management options (6,7). The purpose of the current review is to provide a background on pain flare and RINV, as well as to discuss the novel advances in their management.
Pain flare
Pain flare can be defined in one of two ways: (I) as an a priori 2-point increase in worst pain score [0−10] when compared to baseline with no decrease in analgesic intake; or (II) as a 25% increase in analgesic intake with no decrease in worst pain score. In addition, to distinguish between pain flare and progression of pain, a practical proviso should require pain score and analgesic intake to return to baseline after the flare (8). Several studies have reported the incidence of pain flare in patients treated with palliative RT to painful bone metastases (2,3,6,7,9-13).
The incidence of pain flare following palliative RT for symptomatic bone metastases was reported in a study of 111 patients from three different cancer centers in Canada (2). The overall incidence of pain flare was 40% during RT and within 10 days following the completion of RT. Of the patients treated with a single 8 Gy, pain flare incidence was 39%, which was comparable to the 41% of patients who sustained pain flare following multiple fraction radiation. The majority of pain flares occurred within days 1−5 during the 10-day follow-up period, with only 20% of pain flares occurring through days 6−10. As more than 1/3 of patients experienced pain flare, health care professionals should be aware of this phenomenon and treat patients accordingly.
Hird et al. conducted a questionnaire interview of 13 patients with pain flare. The authors found that pain flare interfered with patients’ daily activities and general functioning (9). Patients also experienced anxiety and worry regarding the success of the treatment. When pain flare occurred, 85% of patients preferred prophylaxis for the management of pain flare rather than an increase in analgesic use, which is associated with adverse events such as dry mouth, drowsiness and constipation (9).
Dexamethasone has been shown to be feasible as a prophylactic agent against pain flare. Two pilot studies, including a phase II study by Hird et al., investigated the use of dexamethasone before treatment for prophylaxis (6,7). The first study included 33 patients who were prescribed 8 mg of dexamethasone before RT and reported that only one patient experienced pain flare in the first 2 days of follow-up (7). In the second phase II study by Hird et al., 62 patients were prescribed 8 mg of dexamethasone just before receiving a single 8 Gy of palliative RT and for 3 consecutive days afterwards (6). Of the 41 patients evaluable, overall incidence of pain flare was 22% with 55% of these flares occurring on day 5. After the completion of RT, the absence of pain flare for days 1−5 was 83% and 95% for days 6−10. The authors concluded that dexamethasone is effective in the prophylaxis of radiation-induced pain flare after palliative RT for bone metastases and recommended that randomized studies should be done to confirm this finding.
Yousef and El-Mashad randomized 120 patients with bone metastases treated with 30 Gy in 10 fractions to a 24-hour infusion of methylprednisone (5 mg/kg) the day before RT or normal saline infusion. Four patients (6.6%) in the steroid arm and 12 patients (20%) in the placebo arm experienced pain flare (P<0.05). The mean duration of the pain flare was 1.25 and 3.75 days, respectively (14). The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) SC23 study is a phase III, double-blind study of dexamethasone vs. placebo in the prophylaxis of pain flare following palliative RT for bone metastases (Chow E, NCT01248585, unpublished data). Patients treated with a single 8 Gy were randomized to receive either 8 mg dexamethasone or placebo for 5 days. The accrual has been completed and we await the analysis of the results.
Radiation-induced nausea and vomiting (RINV)
RINV was first described in 1953 as an acute syndrome by Brown (15) and further explained as such by Danjoux et al. in 1979 (16). As RINV was initially defined as an acute syndrome, relatively few trials have reported the presence of delayed or prolonged emesis (17). Recognizing this, Presutti et al. published a study in 2010 that sought to report the pattern of nausea and vomiting in patients in the moderate risk group receiving prophylaxis with a 5-HT3 receptor antagonist while undergoing palliative RT for painful bone metastases (17). They defined the acute phase as the beginning of RT up until 24 hours after completion of RT, and the delayed phase as 24 hours after the completion of RT up to 10 days following RT. From the acute to delayed phase, complete control of nausea was observed to decline from 54% to 46% in the single-fraction group and from 67% to 50% in the multiple-fraction group. In addition, complete control of vomiting declined from 92% to 62% in the single-fraction group and from 67% to 50% in the multiple-fraction group. The authors noted that RINV may occur up to 10 days post-RT.
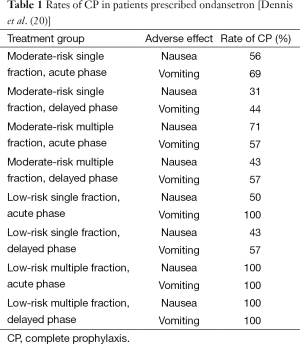
The clinical significance of the delayed phase has been further illustrated in several studies. In a study demonstrating the prevalence of RINV in the delayed phase, Dennis et al. prefaced their paper by noting that if deprived of prophylactic treatment, approximately 50−80% of patients undergoing RT would experience symptoms of nausea and vomiting (18). However, Dennis et al. showed that even with the aid of prophylaxis, RINV continues to be common among patients undergoing palliative RT for bone metastases (18). In a study designed to investigate the incidence and timing of RINV in bone metastases patients receiving prophylaxis with a 5-HT3 receptor antagonist, Dennis et al. prescribed ondansetron to 59 patients and had them document episodes of nausea and vomiting in daily dairies before and during RT, and up until 10 days after completion of RT. To determine the incidence and timing of nausea and vomiting, rates of complete prophylaxis (CP), defined as no nausea or vomiting events and no rescue medication (19), were calculated for the acute and delayed phases. These CP rates are summarized in Table 1. Despite prophylaxis with 5-HT3 receptor antagonists, RINV was common, especially in the delayed phase.
RINV is among the most common adverse effects of RT and is often the first clear sign of radiation toxicity (5). Despite its clinically important effect of potentially decreasing compliance with treatment (21), RINV continues to be underestimated by radiation oncologists (22).
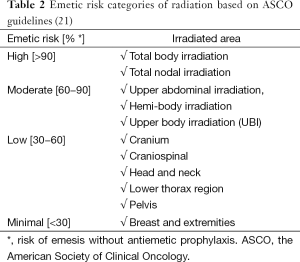
The Multinational Association for Supportive Care in Cancer (MASCC) and the European Society for Medical Oncology (ESMO) have formulated guidelines for categorizing the risk of emesis due to RT (23). These guidelines have been endorsed by the American Society of Clinical Oncology (ASCO) and are divided into four categories (23). Table 2 summarizes the emetic risk categories of radiation.
Serotonin (5-hydroxytryptamine; 5-HT) 5-HT3 receptor antagonists are the first class of antiemetic drug designed specifically to prevent against radiation-induced emesis (RIE) (24). 5-HT3 receptor antagonists inhibit emesis through the action of 5-HT. Specifically, they block the site of 5-HT3 receptors on the vagus nerve in the gastrointestinal tract as well as in the section of the brain dedicated to emesis (19). The effectiveness of 5-HT3 receptor antagonists in treating RINV has been well established (25). However, prior to a randomized trial published in 2006 by Wong et al. (25), the role of dexamethasone alone or in combination with 5-HT3 receptor antagonists was not well-defined. Co-ordinated by the NCIC CTG, Wong et al. (25) conducted a placebo-controlled randomized trial of 211 patients to assess the efficacy of prophylactic dexamethasone for the control of RIE when added to ondansetron during days 1−5 of fractionated RT. The study tested whether ondansetron and dexamethasone could provide superior control of RIE over ondansetron alone during the prophylactic period, and whether the combination could provide sustained control of RIE during subsequent fractions of RT. They found a trend for improved complete control of nausea in the dexamethasone arm during the prophylactic period (50% vs. 38%; P=0.06). In addition, patients treated with dexamethasone had statistically significant benefit in terms of complete control of emesis (23% vs. 12%; P=0.02) and had a lower average nausea score (0.28 vs. 0.39, P=0.03) during the overall study period. Dexamethasone is a potentially useful addition to the 5-HT3 receptor antagonists in the RT setting.
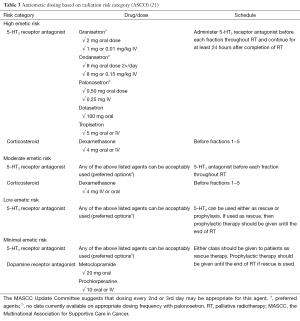
In their recent practice guideline update for antiemetics published in 2011, ASCO acknowledged the findings of Wong et al. (25) and accordingly recommended that patients be offered a short course of dexamethasone during fractions 1−5 as the optimal prophylaxis option for nausea and vomiting caused by moderate emetic risk RT (23). A summary of their full recommendations for antiemetic dosing by radiation risk category is found in Table 3.
With the acknowledgement of 5-HT3 receptor antagonists as effective antiemetics in the prophylaxis of RINV, further studies have been undertaken to examine the comparative efficacy of specific 5-HT3 receptor antagonists as well as their efficacy when used in combination with aprepitant, a substance P neurokinin 1 receptor antagonist (NK1-RA). Specifically, in a paper published in 2014, Dennis et al. completed a pilot study to evaluate, for the first time, the efficacy of a combination of aprepitant and granisetron (a 5-HT3 receptor antagonist) in patients suffering from RINV after receiving moderately-emetogenic RT for thoracolumbar bone metastases (20). This combination had been shown to be effective in the setting of chemotherapy-induced nausea and vomiting (CINV) (26-29), however, prior to Dennis et al.’s study; no published studies had assessed the efficacy of this combination in a RINV setting (20). In their two-armed, nonrandomized prospective pilot study, Dennis et al. found control rates for single-fraction patients (n=13) to be 100% for acute nausea, 62% for delayed nausea, 100% for acute vomiting and retching, and 85% for delayed vomiting and retching. In addition, control rates for multiple-fraction patients (n=6) were 67% for acute nausea, 83% for delayed nausea, 67% for acute vomiting and retching, and 83% for delayed vomiting and retching. The combination of aprepitant and granisetron produced symptom control rates that were numerically superior to those observed in well-matched historical control patients receiving prophylaxis with a 5-HT3 receptor antagonist alone.
Further evidence has since favored the use of aprepitant in combination with granisetron. A case report published by Rowbottom et al. also described the efficacy of aprepitant prescribed in conjunction with granisetron for a patient who had failed ondansetron in the prophylaxis of RINV (30). In this case, a 47-year-old female patient with extensive bone metastases to the spine from breast cancer was initially prescribed ondansetron as an antiemetic after RT. After unsuccessful prophylaxis in which she experienced severe nausea and emesis, the patient was switched to a course of granisetron and aprepitant. This treatment proved efficacious, and the patient completed the remainder of her radiation treatment with no further emesis and minimal nausea.
Even though some 5-HT3 receptor antagonists, such as ondansetron, have mainly been prescribed in the form of oral pills (31), patients in the palliative setting may suffer from co-morbidities such as dysphagia that can make it difficult to administer pills orally. As such, rapidly dissolving film (RDF) formulations have been created, and ondansetron has been produced as a dissolvable film formulation (Ondissolve). Wong et al. conducted a prospective pilot trial to investigate the efficacy of Ondissolve in patients receiving emetogenic radiation (32). The 30 patients in the study were categorized into primary or secondary prophylaxis groups, with primary prophylaxis patients not having pre-existing emetic episodes. In the primary prophylaxis group, the overall control rates during the acute phase for nausea and vomiting were 89% and 93%, respectively; in contrast, the control rates were 73% and 75% for nausea and vomiting in the delayed phase, respectively. In the secondary prophylaxis group the overall control rates for both nausea and vomiting were 100% for the acute phase, and 50% for the delayed phase. The authors concluded that Ondissolve is effective in the prophylaxis of RINV. Randomized trial of ondansetron vs. Ondissolve may be needed as control rates were higher than numerical values historically reported for ondansetron.
Popovic et al. sought to compare, through meta-analysis in the chemotherapy setting, the efficacy of palonosetron compared with other 5-HT3 receptor antagonists (33). Palonosetron is a new generation 5-HT3 receptor antagonist characterized by a longer plasma elimination half-life (about 40 vs. 5.7 hours for the half-life of ondansetron) and highly-selective binding affinity to the 5-HT3 receptor (33). Of the 16 randomized controlled trials identified, 2,896 patients were randomized to palonosetron and 3,187 were randomized to other 5-HT3 receptor antagonists for the prophylaxis of CINV. They found palonosetron to be consistently statistically superior in measures of complete response, complete control, no emesis, no nausea, and sometimes in no rescue medication. Palonosetron was also found to be statistically significantly safer in dizziness and mean QTc interval change. Palonosetron is safer and more efficacious than other 5-HT3 receptor antagonists (33). A phase II prospective study of palonosetron in the prophylaxis/rescue of RINV is ongoing in Canada (Chow E, NCT02388750, unpublished data).
The use of a granisetron transdermal delivery system (GTDS) patch in the CINV population with moderately or highly emetogenic multi-day chemotherapy has been proven effective (34). A GTDS patch is an 8×6 cm clear plastic-backed patch with an adhesive layer containing 34.3 mg of granisetron (34). GTDS provides continuous delivery of granisetron over a period of 7 days, and provides similar exposure to an oral dose of 2 mg a day (35). As such, it enables a convenient option for sustaining antiemetic administration throughout a multi-day chemotherapy regimen and may also be beneficial in a radiation setting. In their double-blind, phase III, non-inferiority study, Boccia et al. (34) compared the efficacy and tolerability of the GTDS to daily oral granisetron. A total of 582 patients were studied, and complete control was achieved by 60% of patients in the GTDS group, and 65% in the oral granisetron group; as such, the GTDS displayed non-inferiority to oral granisetron. Investigation of the efficacy of a GTDS patch in the radiation setting may yield similar findings.
Another finding from chemotherapy-based prophylaxis studies that might be interesting to investigate in a radiation setting was described by Grote et al. (36) in a phase II, open-label study. They investigated the safety and efficacy of palonosetron given in conjunction with dexamethasone and aprepitant in the prophylaxis of CINV. Of the 58 patients evaluable, 88% had a complete response in the acute phase, and 78% in the delayed phase. Moreover, greater than 90% of patients during all time intervals had no emetic episodes, leading the authors to conclude that this particular regimen to be a safe and highly efficacious course of prophylaxis for CINV. Few patients were found to have adverse events considered by the investigators to be related to study medication, however, the most common events were constipation (21% of patients), diarrhea (17%), fatigue (16%), insomnia (14%), and thrombocytopenia (10%) (36). Given the similar underlying mechanism of RINV and CINV, this combination of palonosetron, dexamethasone, and aprepitant may also be efficacious in a radiation setting and should be further investigated.
Finally, a novel oral fixed dose combination of a new NK1-RA called netupitant plus palonosetron (NEPA) has been shown to provide superior prophylaxis of CINV when compared with palonosteron alone (37,38). A randomized phase III published by Aapro et al. evaluating the safety and efficacy of NEPA compared the efficacy of a single oral dose of NEPA vs. a single oral dose of palonosetron alone (38). In a population of 1,455 patients receiving chemotherapy, the authors discovered that the percentage of patients with complete response in the delayed phase was significantly higher in the NEPA group when compared with the palonosetron group (76.9% vs. 69.5%; P=0.001). In addition, the percentages of patients with complete response in the overall (0−120 hours) and acute phases (0−24 hours) were also significantly higher in the NEPA group: 74.3% vs. 66.6% (P=0.001) and 88.4% vs. 85.0% (P=0.047) respectively. As all patients also received a dose of dexamethasone, Aapro et al. concluded that NEPA plus dexamethasone were superior to palonosetron plus dexamethasone in preventing CINV. Again, whether or not this finding can be reproduced in a RINV population would be an interesting study objective to achieve.
Conclusions
The phenomena of pain flare, nausea and vomiting after RT, are important considerations for health care professionals involved in the supportive treatment of patients undergoing palliative RT. The current report sought to provide a background on developments in the area of these two adverse-effects, and to provide an update on recent advances in the field. We recommend that antiemetic prophylaxis be given based on emetic risk category as outlined in the ASCO guidelines. In dealing with RINV, we recommend that further research place greater attention on different prophylactic treatments investigated in chemotherapy studies, and to reproduce such studies in a radiation setting in order to assess whether similar findings might apply. At present, there are no guidelines for the use of pain flare prophylaxis. Further research in this area is needed.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chow E, Wong R, Hruby G, et al. Prospective patient-based assessment of effectiveness of palliative radiotherapy for bone metastases. Radiother Oncol 2001;61:77-82. [PubMed]
- Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three canadian cancer centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [PubMed]
- Chow E, Ling A, Davis L, et al. Pain flare following external beam radiotherapy and meaningful change in pain scores in the treatment of bone metastases. Radiother Oncol 2005;75:64-9. [PubMed]
- Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. [PubMed]
- Young RW. Mechanisms and Treatment of Radiation-Induced Nausea and Vomiting. In: CJ Davis, GV Lake-Bakaar, DG Grahame-Smith, editors. Nausea and vomiting: mechanisms and treatment. Heidelberg: Springer Berlin Heidelberg, 1986;3:94-109.
- Hird A, Zhang L, Holt T, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol) 2009;21:329-35. [PubMed]
- Chow E, Loblaw A, Harris K, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a pilot study. Support Care Cancer 2007;15:643-7. [PubMed]
- Chow E, Wu JS, Hoskin P, et al. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol 2002;64:275-80. [PubMed]
- Hird A, Wong R, Flynn C, et al. Impact of pain flare on patients treated with palliative radiotherapy for symptomatic bone metastases. J Pain Manag 2009;2:401-6.
- McDonald R, Chow E, Rowbottom L, et al. Incidence of pain flare in radiation treatment of bone metastases: A literature review. J Bone Oncol 2014;3:84-9.
- Harris K, Chow E. Need for better documentation and definition of pain flare following external beam radiotherapy in the treatment of bone metastases. J Pain Symptom Manage 2007;33:6-8. [PubMed]
- Chiang A, Zeng L, Zhang L, et al. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:638-42. [PubMed]
- Agarwal A, Popovic M, Lechner B, et al. Rapid onsets of pain flare and pain relief following palliative radiotherapy in a patient with bone metastases. J Pain Manag 2014;7:89-93.
- Yousef AA, El-Mashad NM. Pre-emptive value of methylprednisolone intravenous infusion in patients with vertebral metastases. A double-blind randomized study. J Pain Symptom Manage 2014;48:762-9. [PubMed]
- Brown WM. Symptomatic disturbance after single therapeutic dose of x rays; its relationship to the general radiation syndrome. Br Med J 1953;1:802-5. [PubMed]
- Danjoux CE, Rider WD, Fitzpatrick PJ. The acute radiation syndrome. A memorial to William Michael Court-Brown. Clin Radiol 1979;30:581-4. [PubMed]
- Presutti R, Nguyen J, Holden L, et al. Radiation-induced nausea and vomiting in a palliative radiotherapy clinic: A preliminary analysis. J Pain Manag 2010;3:301-7.
- Dennis K, Nguyen J, Presutti R, et al. Prophylaxis of radiotherapy-induced nausea and vomiting in the palliative treatment of bone metastases. Support Care Cancer 2012;20:1673-8. [PubMed]
- Naylor RJ, Rudd JA. Mechanisms of chemotherapy/radiotherapy-induced emesis in animal models. Oncology 1996;53 Suppl 1:8-17. [PubMed]
- Dennis K, De Angelis C, Jon F, et al. Aprepitant and granisetron for the prophylaxis of radiotherapy-induced nausea and vomiting after moderately emetogenic radiotherapy for bone metastases: a prospective pilot study. Curr Oncol 2014;21:e760-7. [PubMed]
- Abdelsayed GG. Management of radiation-induced nausea and vomiting. Exp Hematol 2007;35:34-6. [PubMed]
- Feyer P, Jahn F, Jordan K. Radiation induced nausea and vomiting. Eur J Pharmacol 2014;722:165-71. [PubMed]
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189-98. Erratum in: J Clin Oncol 2014;32:2117. [PubMed]
- Tramèr MR, Reynolds DJ, Stoner NS, et al. Efficacy of 5-HT3 receptor antagonists in radiotherapy-induced nausea and vomiting: a quantitative systematic review. Eur J Cancer 1998;34:1836-44. [PubMed]
- National Cancer Institute of Canada Clinical Trials Group (SC19), Wong RK, Paul N, et al. 5-hydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: a placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol 2006;24:3458-64. [PubMed]
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090-8. [PubMed]
- Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomized clinical trials. Cancer 2005;104:864-8. [PubMed]
- Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112-19. [PubMed]
- Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822-30. Erratum in: J Clin Oncol 2005;23:5851. [PubMed]
- Rowbottom L, Pasetka M, McDonald R, et al. Efficacy of granisetron and aprepitant in a patient who failed ondansetron in the prophylaxis of radiation induced nausea and vomiting: a case report. Ann Palliat Med 2015;4:32-4. [PubMed]
- Salvo N, Doble B, Khan L, et al. Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: a systematic review of randomized trials. Int J Radiat Oncol Biol Phys 2012;82:408-17. [PubMed]
- Wong E, Pulenzas N, Bedard G, et al. Ondansetron rapidly dissolving film for the prophylactic treatment of radiation-induced nausea and vomiting-a pilot study. Curr Oncol 2015;22:199-210. [PubMed]
- Popovic M, Warr DG, Deangelis C, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 2014;22:1685-97. [PubMed]
- Boccia RV, Gordan LN, Clark G, et al. Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 2011;19:1609-17. [PubMed]
- Howell J, Smeets J, Drenth HJ, et al. Pharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomiting. J Oncol Pharm Pract 2009;15:223-31. [PubMed]
- Grote T, Hajdenberg J, Cartmell A, et al. Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol 2006;4:403-8. [PubMed]
- Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 2014;25:1340-6. [PubMed]
- Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328-33. [PubMed]