Early palliative care and its translation into oncology practice in Canada: barriers and challenges
Introduction
Each year, nearly 200,000 Canadians develop cancer, resulting in approximately 40% of Canadians that will be diagnosed with cancer during their lifetimes. Over 800,000 Canadians have been diagnosed with cancer during the previous 10 years. Although the 5-year relative survival ratio for people diagnosed with cancer has increased to 63%, in 2014 76,600 cancer deaths have been registered (1). Cancer treatment in Canada, as elsewhere, has evolved from tumour-based treatment to person-centered care, addressing not only the physical but also substantive psychological, social, emotional and practical sufferings faced by patients and their caregivers. This shift in practice is entirely convergent with the goals of palliative care, “an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” (2). Service organization and delivery has likewise evolved from sequential care to articulated recommendations for early, integrative care (3-8).
Canadian oncology practice is influenced by international clinical practice guidelines (CPGs) and will be described in the first section. The second section focusses on the barriers and facilitators which describe factors and their mutability. Thirdly, we have seen that Canadian individuals, programs and organizations have themselves innovated and serve as examples of best practices for others to adopt. Eight of these are discussed in greater detail. Opportunities to address the remaining gaps and next steps conclude this survey article.
Canadian CPGs and accreditation standards
A historical focus on sequential care continuity focussed on the seamless transition between oncological care and palliative care (9). We now believe that early identification and treatment of symptoms are best accomplished through convergent, integrative or collaborative practices between the two disciplines. Ideally, these combined services are co-located in comprehensive cancer centres from the time of diagnosis forward and characterized by frequent communication and coordination of care.
Integrating palliative care early during the cancer patients’ trajectory requires a substantive change in clinical practice. Addressing entrenched attitudes and changing physician behaviour is a social process and is typically accomplished through CPGs. These guidelines, in turn, are the result of resource-intensive and consensus-driven processes. Their influence is both indirect and direct. In the field of oncology, organizations such as American Society of Clinical Oncology (ASCO) and National Cancer Comprehensive Network (NCCN) influence Canadian practice indirectly through the participation of Canadian oncologists. In 2014, for example, ASCO was featured at 55 educational events in 33 countries, reaching nearly 12,000 participants (10). In the same year, over 4 million copies of the NCCN’s 60 CPGs were downloaded. Of these, over half were assessed by members outside the United States. NCCN influenced practice of roughly 20,000 individuals through 175 educational events (11). These two organizations differ fundamentally, however, in the development of their guidelines. ASCO largely relies on an evidence-based approach, whereas NCCN is driven by the opinions of clinical experts. Encouraged by these organizations, methods and tools exist to directly influence clinicians through the local adaptation and implementation of CPGs from organizations such as ASCO and NCCN (12). In Canada, the Canadian Partnership Against Cancer (CPAC) developed CAN-ADAPTE and CAN-IMPLEMENT, a set of processes and guidelines to facilitate the national and provincial uptake of internationally derived guidelines (13).
ASCO’s provisional clinical opinion regarding the integration of palliative care into standard oncology care in 2012 marked a key turning point in this discussion (14). Although based on strong evidence from a single study (15), ASCO’s recommendation resonate with palliative care leaders and programs throughout the country as evidenced by a number one recommendation by both the Canadian Medical Association and the Canadian Society for Palliative Care Physicians: “Don’t delay palliative care for a patient with serious illness who has physical, psychological, social or spiritual distress because they are pursuing disease-directed treatment” (16). Concurrently, ASCO’s leadership development program charged a working group with researching and developing a commensurate service delivery model (17). Their research identified the Cancer Care Ontario Program in Evidence-Based Care and their adoption of palliative care integration as an exemplar, recommending, adoption in the US with only “minor modifications”. The NCCN subsequently published a comprehensive set of palliative guidelines in 2015 with focus on screening, assessment, interventions, reassessment and after-death care (18,19).
Although ASCO focussed on a single study, a growing evidence base has been surveyed by others (8,20-23). In addition to the Temel study (15,24), the Education, Nurture, Advise, Before Life Ends (ENABLE) II study sought to understand physician perspectives of routine integration of palliative care into oncology practice based on a model developed in 1999 (25). Both sets of studies defined early intervention as assessment, symptom management, communication, education and follow-up on a monthly basis. Minor differences emerge regarding the role of the nurses and physicians. Zimmerman and colleagues conducted a similar study involving a larger randomized clinical trial in Toronto Canada (26). Outcomes across all three studies in terms of functional status, satisfaction, quality of life and symptom management are largely confirmatory in nature. A series of observational studies by Bruera and colleagues are noteworthy in that they were modelled after the Canadian comprehensive model (27-30).
The Multinational Association of Supportive Care in Cancer (MASCC) is yet another international multidisciplinary organization which emphasises research and education of supportive care throughout the disease trajectory. The focus of the organization is communication and integration between interdisciplinary members. In addition to medical oncology, surgical oncology and radiation oncology physicians, their target audience includes “nurses, dentists, dental hygienists, pharmacists, social workers, dietitians, outcomes specialists, psychologists, statisticians, infectious disease specialists, educators, representatives from industry and non-profit sectors” (31).
Canada-specific guideline development focussed on early screening for distress. Sponsored by CPAC, the Canadian Journey Action Group was commissioned to conduct a review of the literature, resulting in the identification of 32 best clinical global practices (32). Following a series of consultations in 2008 and 2009, a toolkit and recommendations were issued (33). Both the Edmonton Symptom Assessment System (ESAS) and Canadian Problem Checklist were recommended as tools to be used early and throughout the cancer trajectory. These recommendations do not include assessment or prescribe specific interventions. Rather, they provide a framework for a comprehensive, population-based and person-centered approach. Annual performance surveys suggest that these recommendations have now been largely implemented across Canada (Figure 1) (34).
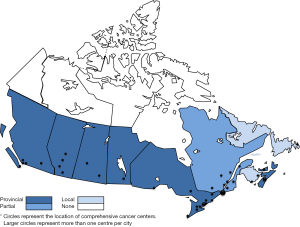
CPAC is a national organization responsible for promoting a national cancer strategy. Guideline development advocating early integration of palliative care also takes place at the provincial and regional health care organization levels. Cancer Care Ontario, the British Columbia Cancer Agency (BCCA) and the Canadian Virtual Hospice (Manitoba) are the leading advocates and have focussed on symptom management (35-37). The Edmonton, Calgary and Fraser Health regions have been instrumental in the introduction of comprehensive, integrated and coordinated programs focussing on assessment and treatment of symptoms. Both Calgary and Fraser Health have developed comprehensive programs focussing on advance care planning (38-40). Significant consensus statements utilizing the ADAPTE framework have been developed for fatigue (41) and sleep disturbances (42).
The voluntary nature of CPGs presents a challenge for their uptake. Relationships between physicians, member organizations, governments and health care provider organizations are complex. Professional autonomy recognizes the special nature of physicians’ experience and knowledge. Education standards have been drafted for medical and radiation oncology specialty training. Health care provider organizations are beginning to develop operational polices which govern individual and program behaviour as in the case of Alberta Health Services’ (AHS) Advance Care Planning Policy. Most importantly in Canada, however, is the foray of governments through setting accreditation standards for health care organizations. Accreditation Canada, for example, has entrenched several guidelines (Table 1). These standards are, however, new, have not been evaluated, and their ability to influence individual physician behaviour is unknown.
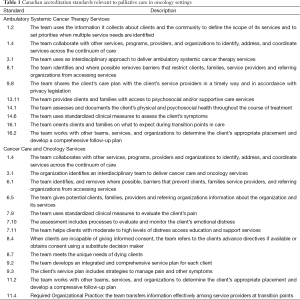
Full table
Barriers and challenges 2000
Integrating palliative and oncologic care in Canada is subject to the same barriers and challenges as have been described elsewhere (43-46). These barriers and challenges are discussed from a Canadian context.
Education and attitudes
First among these is what Abrahm and colleagues (47) refer to as “learned helplessness” or simply the lack of training and experience. Subspecialty training for palliative care is currently under development in Canada. Currently, a 1-year residency program is offered, jointly accredited by the Royal College of Physicians and Surgeons of Canada and the College of Family Physicians of Canada. The added competency in palliative medicine is designed to support practice in both academic and community settings, principally as a consultant. The number of oncology residents enrolled in palliative medicine residency programs is not currently known. Commensurate experience, managing a large caseload and dedicating 50% of one’s time to palliative practice however describes clinicians trained prior to the palliative residency programs being available.
Additional educational platforms provide palliative content: Education in Palliative and End-of-life Care (EPEC) and Learning Essential Approaches to Palliative and End-of-Life Care (LEAP). Both contain oncology modules. However, the lack of systematic funding means that their uptake by oncologists has been limited.
Training notwithstanding, oncologists may also obtain knowledge of palliative care through collaboration with palliative care programs and their clinicians. Screening, assessment and treatment of symptoms alongside the development of appropriate prognostication and communication skills can be learned in this manner. There are 42 major cancer centres across Canada. However, a register and descriptive data of Canadian centres do not exist, regarding their co-location and integration with palliative care. Anecdotally, we are told that patients across the majority of centres have access to palliative outpatient and consultative care. We are also told that very few centres are associated with inpatient palliative care units, consistent with what had been identified in a US survey (6).
Compassion fatigue
Continually faced with suffering and death, oncologists are also prone to “compassion fatigue” and burnout (47). According to Abrahm and colleagues, compassion fatigue manifests itself as stress, depression, anxiety, substance abuse and family disruption. More importantly, however, this behaviour is associated with avoiding much needed discussions and referrals. Prolonged and unresolved stress can result in more complicated disorders and eventual burnout. Although financing and organization of health care in Canada are unique, there is no reason to believe that Canadian oncologists experience less detachment and depersonalization.
Difficulty communicating goals of care
It is common for newly diagnosed metastatic patients receiving chemotherapy to hope for a cure (48). Motivation for the oncologist to discuss the relative effects on survival versus symptom control may be limited. Chemotherapy itself can be prescribed as a means to maintain hope. Only 10% of Canadians discuss end-of-life treatment preferences with their physician (49). Without appropriate discussion and goals setting, the oncologist may inadvertently delay referral to palliative care. Appropriate advance care planning and patient education are initiatives that purport to address these barriers.
Barriers also reflect patient and population perspectives. Ipsos-Reid conducted a poll to find that, although 44% of Canadians discussed end-of-life care with their families, only 9% of Canadians have had similar discussions with their physicians (50). A decade later, the proportion of Canadians discussing their end-of-life preferences with their physicians remained constant (51). Additional questions probed the importance of these discussions with 61% of those surveyed, acknowledging that these discussions need to take place with at least one individual. Slightly more than half (52%) provided the support for early discussions, i.e., when the patient is healthy. Once again, the concept of early integration was favored by the majority of Canadians in a recent Harris/Decima poll (52). The poll was conducted for the Canadian Hospice Palliative Care Association (CHPCA) for their “Way Forward” national initiative. Specifically, the majority of Canadians (73%) expect these discussions to take place with their physicians, preferably when they are healthy or when they are diagnosed with a life-limiting disease (80%).
Terminology
The term “palliative care” was coined in Canada through the establishment of an acute unit within the Royal Victoria Hospital in Montreal, Quebec (53). As in other jurisdictions, the association of the word “palliative” with dying in Canada serves as a barrier to referral and treatment of symptoms. In fact the development of the term “supportive care” resulted from the need to manage symptoms in patients on cancer treatment (54). Evidence in fact supports the notion that oncologists are more likely to refer to a supportive care service as opposed to a palliative or hospice care service (55).
Paucity of research activity
The lack of research infrastructure and funding, lack of consensus on appropriate outcomes and ethical issues describe challenges that originate with palliative care research in Canada. Approximately $8M per year was invested by Canadian funders between 2005 and 2010 (56). Because funding palliative care research had become a priority only recently, approximately one-third of these research dollars were targeted to increasing capacity and infrastructure. This investment represents approximately 1% of all cancer research investment during this time. As a result, the vast majority of research dollars continue to be directed toward prevention, early detection and treatment. Although prioritized funding for palliative care is laudable, the relative paucity of research being conducted in Canada can be interpreted as a barrier to provide and translate knowledge from the perspective of a busy oncologist.
Aggressive cancer care
Aggressive cancer care is antithetical to early palliative care. The term aggressive refers to a style of oncology practice which is focussed on treatment of cancer as opposed to the symptoms. Late or no referral to palliative care and excessive use of health care resource use characterize this phenomena. A retrospective study of administrative data in Ontario, Canada between 1993 and 2004 examined the situation in Canada and compared the results to the US (57). Aggressive care was found in 22.4% of the 227,161 patients. More importantly, aggressiveness was shown to be increasing each year and was shown to be approaching US practice patterns. The definition of aggressiveness, however, is controversial. Firstly, difficulties in prognostication make it difficult to prospectively time treatment and foresee sentinel events such as hospital-acquired infections. Secondly, administrative data does not include the intent of treatment. A strong argument can be made that all chemotherapy and radiotherapy in the presence of metastatic disease is in fact palliative in nature.
Organizational and operational characteristics
Financing and delivery of health care in Canada falls within the provincial domain. Organizations such as the Canadian Cancer Society and the CPAC serve as vehicles to raise funding and awareness within the context of a tightly managed economy with scarce and competing resources. These organizations also play an important role in knowledge translation and help to make innovative practices and research more broadly available. Cancer control describes all polices and operations within each province to prevent, diagnose and to treat cancer and it sequelae. Cancer control however remains siloed and not integrated with other institutions within the health care system. Understanding this requires a brief introduction to regionalization.
Canadian provinces are further defined by health care regions or zones. These are administrative units with varying degrees of strategic, budgetary and operational control. In most provinces, cancer control is administered equivalently to an autonomous health region. While this allows for the geographic standardization of resource deployment, it has the unintended effect of isolation from other institutions. Institutional and regional policies and procedures are for the most part not integrated and coordinated. We speculate that the clinical and front line staff are likewise hampered by these administrative factors.
In Alberta, innovative quasi-administrative units called Strategic Clinical Networks (SCN) exist to help break through the siloed administrative barriers. The Cancer SCN brings together stakeholders from across the province from prevention, health care, research, and policy to lead and support evidence-informed improvements and bring innovation to health care. One of their initiatives includes early diagnosis and management of lung cancer. Predominantly targeting wait times, the cancer SCN can work with the surgery and the respiratory SCNs to address barriers to the early diagnosis and management of lung cancer. A second initiative is working toward the establishment of standardized province-wide referral and triaging process that will improve access and decrease wait times (38).
Acceptance criteria for referral and service by palliative care programs often include expected survival, a do-not-resuscitate order and an absence of chemotherapy treatment. These criteria are employed to limit the demands of relatively scarce palliative care resources. Palliative chemotherapy and radiotherapy are however entirely consistent with appropriate end-of-life treatment for cancer patients and consistent with best practices. The prevalence of these interventions and their reference in admission and discharge criteria are not known.
Co-location of programs increases the likelihood of communication between programs. However, patients in most centres in Canada move between facilities for in- and out-patient care. Of the 42 cancer centres in Canada, most are outpatient facilities. Inpatient cancer treatment largely takes place in neighbouring acute care facilities. Although more than half of the acute care facilities have designated wards and beds for cancer services, many do not. Furthermore, co-location and availability of palliative care programs are variable. Notable exceptions are innovative and described in more detail in the next section.
Opportunities and innovation
Increasing attention and resources to integrated care between palliative and oncology programs is evidenced globally. A number of high quality studies have demonstrated the benefits of integrated palliative care programs in major cancer centres, implementation of integrated palliative care and implementation of specific palliative interventions (58). In their narrative review, Debono and colleagues describe the experience of both the MD Anderson Cancer Center and Princess Margaret Cancer Centre (PMCC) groups. PMCC’s promising phase II study demostrated improvements in both symptom control and family satisfaction (59). In addition to the well known Temel and ENABLE studies, the review includes a synthesis and discussion of several smaller trials. Early pilot and phase II studies have been shown to be validated by phase III studies. Evidence and interventions are not limited to the organizational level but are also aimed at oncologists. A relatively recent narrative review was written to support the role of oncologist as providing palliative care services (60). The review focused on communication, symptom assessment, preparing advance directives, help with spiritual needs, and facilitating early referrals to hospice care.
In 2013, the Government of Canada, provided the CHPCA with $3M dollars to support the development of a national framework promoting the adoption of integrated palliative care. Following the publication of a Roadmap, several discussion papers were completed, including a discussion of the palliative approach, an overview of three models to integrate palliative care into chronic care, and an overview of innovative integrated programs (61). The latter discussion paper highlights innovative practices across entire health regions in Edmonton and Fraser Health. The Edmonton model describes integrated, coordinated and comprehensive care to provide care options at all times within patients’ trajectories and is described in detail elsewhere (62).
Although integration at a regional level supports integrated cancer care, it is not specific to oncology. Specific innovations arising from the integration of palliative and oncological care in Canada are described by the following eight vignettes.
- BCCA Pain and Symptom Management/Palliative Care Program and Family Practice in Oncology Network. The BCCA is an example of a provincial organization dedicated toward comprehensive and integrated cancer control (63). Founded in 1990, it has a broad-based mandate including all elements of cancer control (prevention, screening, diagnosis, treatment, rehabilitation/supportive care and palliation), as well as research (basic, translational, clinical and epidemiological), and education (professional, technical, patients and public). Services are delivered through a geographically-distributed network, including regional cancer centers, community cancer centers, services and clinics, and unifying provincial programs in radiation, systemic and surgical oncology. Connection is made through standards, programs, processes and support systems. The Pain and Symptom Management/Palliative Care Program was developed by a network of care providers from within and outside of the BCCA. It is provincial in scope, with interdisciplinary consultative teams and clinics at each of the six regional cancer centers. The cancer centers are also formally linked with palliative care providers in their catchment area to ensure that care planning is seamless and that opportunities for collaborative projects are fully realized.
BCCA also provides family physicians with enhanced skills in the care of cancer patients, including palliative care. The BCCA Family Practice Oncology Network is believed to be the most comprehensive program of its kind in Canada (64). It consists of four main components: (i) an eight week training program targeted at family physicians outside of urban areas; (ii) cancer care guidelines customized to the needs of family physicians; (iii) continuing education opportunities; and (iv) a twice yearly Journal of Family Practice Oncology. The aim is for all BC communities with 15,000 people or more to have at least one family physician that has completed the training program and can support all aspects of cancer care for local patients (65). - Multidisciplinary Pain and Symptom Control Clinic and Telehealth Clinic. The Multidisciplinary Pain and Symptom Control Clinic was established in 1995 at the Cross Cancer Institute, a tertiary cancer centre in Edmonton. It is designed to address the needs of cancer patients with complex physical and psychosocial-spiritual concerns, by providing consultation with a team of health care professionals with expertise in palliative care and oncology. Patients are scheduled for half-day appointment. The make-up of the team is tailored according to each patient’s specific needs. Members include palliative care physicians, nurse, pharmacist, dietician, physical therapist, occupational therapist, speech language pathologist, respiratory therapist, psychologist, social worker and spiritual care professional. After discipline-specific assessments and team conference are completed, a comprehensive and individualized plan of care is formulated. Elements of the plan that are accepted by the patient are implemented, and care is coordinated with other providers (e.g., family physician, home care team). Patients are followed by telephone or in person until symptoms stabilize, at which time responsibility for symptom management is transferred back to the family physician. In a retrospective evaluation of 166 consecutive patients referred to the clinic, improvement was seen in overall symptom distress, depression, anxiety, wellbeing, and pain. Patients expressed a high level of satisfaction with the clinic (66).
In 2007, a grant was received from the Ministry of Health to offer the clinic to patients living in rural areas of the province of Alberta, using videoconferencing. Rural-based nurses with expertise in oncology or palliative care were provided with training in symptom assessment and management, physical examination, team consultation and use of videoconferencing equipment. On the day of the clinic visit, the patient presents to a rural healthcare facility and undergoes assessment by a nurse. A videoconference link with the multidisciplinary team at the Cross Cancer Institute is established, and team members interview the patient. Following a team conference, recommendations are discussed with the patient, and then sent to the family physician. Evaluation of 44 consultations showed improvement in appetite and anxiety scores, savings to patients in terms of travel distance, time and cost, compared to attending the Cross Cancer Institute for the service, and a high degree of satisfaction on the part of patients and referring physicians. The clinic has since received ongoing operational funding from the provincial health authority (67). - Provincial Palliative Care Integration Project (PPCIP). Based on the recommendations made by national organizations regarding screening for distress, and a pilot project in Southwester Ontario, the PPCIP represents an important Canadian innovation describing a population-based screening and assessment initiative (68). The project itself describes three components: (i) implementation of ESAS for symptom screening and Palliative Performance Scale (PPS) for functional assessment; (ii) manage data through use of quality improvement processes; and (iii) specific improvements to access and integration of palliative care services. Approximately 30% of cancer patients were screened and demonstrated significant improvements in symptom control within the first year, 2006, of implementation.
In Ontario, the Kingston, Frontenac, Lennox and Addington Palliative Care Integration Project initiated a collaborative communication plan demonstration project in 2001 (69). The development of evidence-based collaborative care plans, use of validated assessment tools, and deployment of the collaborative care plans were intended to provide continuity across the continuum of care. Collaborative care plans came into fashion a decade earlier and are characterized by documentation of explicit interventions and expected outcomes. Both the ESAS and PPS were employed. These collaborative plans served as a communication tool between providers in different settings. Although the results from this evaluation were modest, the authors noted a significant increase in the documentation of symptoms and the lack of standardized documentation and definitions of palliative care across study sites.
A key element of this innovation is the Interactive Symptom Assessment and Collection (ISAAC), a touch screen kiosk allowing patients to enter their ESAS scores (70). Patients are also able to enter their scores from their home computers. Clinicians within the patient’s circle of care are then able to access this data in histogram format. Alerts are provided when scores fall within the moderate and high levels. A series of nine recommendations comprised additional specific improvements to the system including: service delivery, education, training, networks, inter- and intra-professional collaboration and bed capacity (71). Monitoring use of standardized screening tools demonstrates that this innovation is being adopted throughout the country at a significant rate (34,72). - Ontario PMCC. The PMCC is Canada’s largest cancer centre and home to an innovative model of integrated comprehensive care comprising clinical, education and research. PMCC is a teaching hospital of the University of Toronto and is affiliated with two academic hospitals (Toronto Western and Toronto General Hospitals). Although the focus is on outpatient care and there is no emergency department, there are 115 inpatient beds. The Department of Psychosocial Oncology and Palliative Care (POPC) was established in 2001. There is an acute palliative unit, daily palliative care clinics, a cancer pain clinic, and both inpatient and outpatient consultation services. A dedicated 12 bed oncology palliative unit and having oncologists work alongside palliative care doctors make this setting unique in Canada (73). PMCC is an integrated research facility for physicians, nurses, social workers and other health care providers. As a research hub, PMCC also functions to generate, implement and evaluate initiatives to integrate palliative and oncology care (73,74).
- Cancer Nutrition and Rehabilitation Clinic. The McGill Cancer Nutrition and Rehabilitation Clinic were founded in 2003 at the Jewish General Hospital in Montreal (75). A second site was later opened at the Royal Victoria Hospital in Montreal, and the model was subsequently replicated at the Elisabeth Bruyere Hospital in Ottawa. Cancer rehabilitation is a process that assists the individual with a cancer diagnosis in obtaining optimal physical, social, psychological, and vocational functioning within the limits created by the disease and its treatment. The premise of the clinic is that nutrition counseling, together with an exercise program and dedicated symptom control, will improve quality of life and functioning in advanced cancer patients. These goals are accomplished through a coordinated multidisciplinary team approach. Eligible patients are those who, as compared with their level before diagnosis, are experiencing changes in appetite (with or without associated weight loss), physical functioning (such as walking), fatigue, and coping with the consequences of their disease. Team members include physicians, nurses, dieticians, physical and occupational therapists, social workers, psychologists, recreational therapists, vocational therapists, case managers, patient coordinators, chaplains and volunteers. After assessment, the team reviews each member’s findings and formulates an individualized treatment plan to meet the patient’s unique and specific situation. Patients undertake bi-weekly exercise sessions with the physiotherapist, and see other team members every 2 weeks. The program lasts for 8-12 weeks, after which patients are referred back to their original physicians. In an uncontrolled prospective intervention study, 188 patients with Stage III and IV cancer were enrolled, 38.2% of whom were on chemotherapy, and 70% completed the program. Patients experienced robust improvements in physical and activity dimensions of fatigue (effect sizes 08-1.1), moderate reductions in severity of weakness, depression, nervousness, shortness of breath and distress (effect sizes 0.5-0.7), and moderate improvements in 6 minutes’ walk distance, maximal gait speed, coping ability and quality of life (effect sizes 0.5-0.7). Seventy-seven percent of patients either maintained or increased their body weight (76).
- Advance Care Planning. Healthcare advances have resulted in a dilemma that profoundly challenges our human values: life can be supported beyond a time when people are capable of speaking for themselves about what care they want to receive. It really matters that patients and their families have conversations about preferences for end-of-life care, and that their healthcare providers are engaged in those conversations. Without these conversations, patients receive more invasive care at the end of life and have worse quality of life (77) and death (78) and their loved ones have worse bereavement adjustment (77). In contrast, patients who do have end-of-life conversations with their physicians are much more likely to receive care that is consistent with their stated wishes (79).
There is a knowledge-to-practice gap in the area of planning for end-of-life care for cancer patients. In 2013, Alberta Health announced a long-range strategic plan for cancer control to take the province through to 2030, with bold initiatives crossing the cancer control spectrum. It emphasizes community-based care and a patient-focused approach, and directs AHS to “Implement a provincial Advance Care Planning process to provide patients and families with the opportunity to define goals for their care”. In concert with Alberta’s Cancer Plan, AHS is leading their provincial counterparts in implementing a multi-year, multi-sector, phased provincial roll-out of a policy for advance care planning. - Rapid Access and Multidisciplinary Palliative Radiotherapy Programs. The first rapid access palliative radiotherapy program in Canada was established at the Odette Cancer Centre in Toronto in 1996. The primary goal is to provide timely palliative radiation therapy for symptom relief in patients with locally advanced or metastatic cancers. Consultation, simulation and treatment planning occur within the same day. Initiation of treatment may occur the same day in urgent cases. In a review of 2,742 patients referred between August 2008 and June 2012, the most common reasons for referral were bone metastases (53%) and brain metastases (21%). A total of 1,890 patients were treated. Median wait time from referral to consultation was 3 days. Sixty percent of patients were treated on the day of consultation, and 33%, within 1 to 6 days (80). Similar programs have been developed in cancer centres across Canada (81-84).
The program at the Cross Cancer Institute in Edmonton has incorporated the additional innovation of providing same-day access to an expanded multidisciplinary team (radiation oncologist, registered nurse, nurse practitioner, radiation therapist, occupational therapist, pharmacist, dietician, social worker), in order to address symptom control and quality of life. During the first year of operation, 82 patients with bone metastases were referred. At 4-week follow-up, intensity of pain, tiredness, depression, anxiety, drowsiness and wellbeing had improved to a statistically significant degree, compared to baseline (85).
At the Tom Baker Cancer Centre in Calgary, a multidisciplinary palliative radiotherapy clinic has been established for patients with brain metastases. In order to optimally support patients in making treatment decisions, the team includes a radiation oncologist, a palliative care physician, a clinical nurse specialist and a spiritual care counselling consultant. A review of the clinic’s first 100 patients revealed that, compared to a historical cohort, the rate of radiotherapy in the last 30 and 14 days of life had decreased from 19% to 9% and from 6% to 1%, respectively (86). - Education Initiatives. Education of oncology professionals in the provision of primary-level palliative care also serves to support integration of the two disciplines. The Royal College of Physicians and Surgeons of Canada certifies physicians to practice in a specialty or subspecialty. Medical Oncology is a subspecialty of Internal Medicine and requires completion of a 2-year residency program. Radiation Oncology is a specialty in its own right and requires completion of a 5-year residency program. In both programs, one month of palliative care training is mandated (87).
Registered nurses in Canada can seek voluntary certification in specialty areas through the Canadian Nurses Association. Certification in Oncology requires demonstration of competency in symptom management and palliation and end-of-life care (88).
CPAC has funded the development, piloting and delivery of a program called EPEC in Oncology (EPECTM-O Canada). It is an adaptation of the American EPECTM-O curriculum, developed for oncologists and other health professionals who provide care to patients with cancer, to the Canadian healthcare context. It is an evidence-based curriculum that uses a ‘train-the-trainer’ mentorship model. As of April 2015, the program has been delivered 22 times, in five provinces, has established 11 senior faculty, 34 local faculty leaders and trained over 850 palliative care and oncology professionals. CPAC is currently seeking an organization that can support and maintain the content, delivery and evaluation of the program, and ensure long-term program success (13).
Pallium Canada is a national education community-of-practice created in 2009 to further the work of the Pallium Project Phases I and II [2001-2007]. It has been funded primarily by the federal government. One of the deliverables of Phase I was the development and implementation of a standardized 2-day interprofessional Palliative Care course for rural family physicians, nurses and pharmacists in the prairie provinces of Alberta, Saskatchewan and Manitoba. The LEAP course employs a train-the-trainer model. In Phase II, the scope of the project was broadened beyond rural regions and included all of Canada. Phase III development of LEAP will introduce tailored modules for palliative care in different settings, including oncology (89).
Remaining gaps and next steps
In this review, we have surveyed the key CPGs and traced their translation into Canadian practice. Guidelines are inherently costly to develop and require frequent updating. Organizations such as ASCO, NCCN and MASCC are therefore sufficiently resourced to influence practice. Canada, as many other countries, relies on the evaluation and translation of evidence. Adoption into local practice is guided through well-established processes. Guidelines are also inherently flexible to accommodate not only variations in local practice, but also variation at the level of the clinician. Complicated relationships exist between the guidelines, medical practice leaders and clinicians within their sphere of influence. In Canada, we have also observed the influence of guidelines on the development of accreditation standards.
Barriers and challenges found in Canadian settings mirror those found elsewhere. Knowledge, skills and attitude all contribute to the behaviours of healthcare providers. Public expectations are determined and shaped through public investments such as public polls and media campaigns. Governments, cancer foundations and educational institutions have been working hard together to educate both the public and healthcare professionals. Significant progress has been made with respect to funding palliative care research in Canada. Nonetheless, this investment represents only 1% of all cancer research in Canada. Emerging evidence suggests that Canadian oncological practice mirrors an aggressive style of care more commonly associated with our neighbours to the south (United States). Terminology and attitudes of the public though may be driving some of the conversations and practices which, in reality, are better described as palliative.
Within the context of these barriers and challenges, numerous innovative practices have been surveyed and have been described. Many of these programs have been newly created and are in the early stages of evaluation. Others are characterized by co-location. Others are characterized through the scope of practice. Comprehensive programs can be defined in many ways spanning time, interventions and settings of care. Specific targeted interventions in addition to process and organizational changes are included.
Although we have learned much from this review, as many questions were raised. These questions require in-depth survey and analysis. A 2-pronged approach is proposed. First, an environmental scan of facilities to determine the exact nature of local palliative and oncologic services, illustrating the characteristics which describe co-location, coordination and integration. Second, a survey of Canadian oncologists using the behavioural domains framework to uncover the factors which help explain current practices from their perspective.
Acknowledgements
We are grateful for the research assistance of Barinder Minhas and Marta Oleszczuk. Various aspects of this review have been informed through discussions with colleagues: Matthew Parliament, Quincy Chu, Jennifer Spratlin and Alysa Fairchild. Finally, we are indebted to the anonymous reviewers.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2014. Toronto (ON): Canadian Cancer Society, 2014. Available online: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2014-EN.pdf
- World Health Organization. WHO Definition of Palliative Care. Available online: http://www.who.int/cancer/palliative/definition/en/, accessed 28 Apr 2015.
- Fann JR, Ell K, Sharpe M. Integrating psychosocial care into cancer services. J Clin Oncol 2012;30:1178-86. [PubMed]
- Ferrell BR, Smith TJ, Levit L, et al. Improving the quality of cancer care: implications for palliative care. J Palliat Med 2014;17:393-9. [PubMed]
- Gaertner J, Weingärtner V, Wolf J, et al. Early palliative care for patients with advanced cancer: how to make it work? Curr Opin Oncol 2013;25:342-52. [PubMed]
- Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA 2010;303:1054-61. [PubMed]
- Shin J, Temel J. Integrating palliative care: when and how? Curr Opin Pulm Med 2013;19:344-9. [PubMed]
- Von Roenn JH. Palliative care and the cancer patient: current state and state of the art. J Pediatr Hematol Oncol 2011;33 Suppl 2:S87-9. [PubMed]
- Glare P, Clarke S. The interface of oncology and palliative care in tertiary hospitals. Cancer Council Australia, 2002. Available online: http://cancerforum.org.au/Issues/2002/March/Forum/The_interface_of_oncology_and_palliative_care_in_tertiary_hospit.htm, accessed 1 May 2015.
- American Society of Clinical Oncology. Available online: http://www.asco.org/, accessed 1 May 2015.
- National Comprehensive Cancer Network. NCCN Annual Report 2014. Available online: http://www.nccn.org/about/annual_report/
- Browman GP, Makarski J, Robinson P, et al. Practitioners as experts: the influence of practicing oncologists “in-the-field” on evidence-based guideline development. J Clin Oncol 2005;23:113-9. [PubMed]
- Canadian Partnership Against Cancer. Available online: http://www.partnershipagainstcancer.ca/, accessed 1 May 2015.
- Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- Choosing Wisely Canada. Palliative Care: Five Things Physicians and Patients Should Question. Canadian Society of Palliative Care Physicians. Available online: http://www.choosingwiselycanada.org/recommendations/palliative-care/, accessed 1 May 2015.
- Partridge AH, Seah DS, King T, et al. Developing a service model that integrates palliative care throughout cancer care: the time is now. J Clin Oncol 2014;32:3330-6. [PubMed]
- Levy MH, Smith T, Alvarez-Perez A, et al. Palliative care, Version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2014;12:1379-88. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Palliative Care. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp, accessed 1 May 2015.
- Bauman JR, Temel JS. The integration of early palliative care with oncology care: the time has come for a new tradition. J Natl Compr Canc Netw 2014;12:1763-71; quiz 1771.
- Ellis JA, McCarthy P, Hershon L, et al. Pain practices: a cross-Canada survey of pediatric oncology centers. J Pediatr Oncol Nurs 2003;20:26-35. [PubMed]
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care - does it work? Support Care Cancer 2012;20:507-13. [PubMed]
- Hui D, Kim YJ, Park JC, et al. Integration of oncology and palliative care: a systematic review. Oncologist 2015;20:77-83. [PubMed]
- Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349-63. [PubMed]
- Bakitas M, Lyons KD, Hegel MT, et al. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013;11:415-23. [PubMed]
- Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. [PubMed]
- Braiteh F, El Osta B, Palmer JL, et al. Characteristics, findings, and outcomes of palliative care inpatient consultations at a comprehensive cancer center. J Palliat Med 2007;10:948-55. [PubMed]
- Bruera E, Hui D. Conceptual models for integrating palliative care at cancer centers. J Palliat Med 2012;15:1261-9. [PubMed]
- Dhillon N, Kopetz S, Pei BL, et al. Clinical findings of a palliative care consultation team at a comprehensive cancer center. J Palliat Med 2008;11:191-7. [PubMed]
- Elsayem A, Swint K, Fisch MJ, et al. Palliative care inpatient service in a comprehensive cancer center: clinical and financial outcomes. J Clin Oncol 2004;22:2008-14. [PubMed]
- Multinational Association of Supportive Care in Cancer. Available online: http://www.mascc.org, accessed 1 May 2015.
- Dunbrack J, Ebrary CEL - York University. Canadian Partnership Against Cancer. Canadian Electronic Library (Firm). eds. Cancer and palliative care: Integration and continuity of care. Toronto: Canadian Partnership Against Cancer, 2009. Available online: https://www.library.yorku.ca/find/Record/3121619/TOC#Descriptio
- Canadian Partnership Against Cancer (CPAC), Cancer Action Journey Group. Guide to Implementing Screening for Distress, the 6th Vital Sign: Moving Towards PersonCentered Care. Part A. Background, recommendations and implementation. Toronto, ON: CPAC, 2009.
- Canadian Partnership Against Cancer. The 2014 Cancer System Performance Report. Toronto (ON): Canadian Partnership Against Cancer, 2014. Available online: http://www.cancerview.ca/idc/groups/public/documents/webcontent/sp_report_2014.pdf
- BC Cancer Agency. A comprehensive cancer control program for BC. Available online: http://www.bccancer.bc.ca/, accessed 1 May 2015.
- Cancer Care Ontario. Available online: https://www.cancercare.on.ca/, accessed 1 May 2015.
- Canadian Virtual Hospice. Available online: http://www.virtualhospice.ca/en_US/Main+Site+Navigation/Home.aspx, accessed 1 May 2015.
- Alberta Health Services. Cancer Strategic Clinical Network. Available online: http://www.albertahealthservices.ca/7677.asp, accessed 1 May 2015.
- Edmonton Zone Palliative Care Program. Available online: http://palliative.org/, accessed 1 May 2015.
- Fraser Health. Available online: http://www.fraserhealth.ca/, accessed 1 May 2015.
- Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233-46. [PubMed]
- Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791-800. [PubMed]
- Davis MP. Integrating palliative medicine into an oncology practice. Am J Hosp Palliat Care 2005;22:447-56. [PubMed]
- Davis MP, Bruera E, Morganstern D. Early integration of palliative and supportive care in the cancer continuum: challenges and opportunities. Am Soc Clin Oncol Educ Book 2013;144-50. [PubMed]
- Dennis K, Librach SL, Chow E. Palliative care and oncology: integration leads to better care. Oncology (Williston Park) 2011;25:1271-5. [PubMed]
- Von Roenn JH. Advance care planning: ensuring that the patient’s voice is heard. J Clin Oncol 2013;31:663-4. [PubMed]
- Abrahm JL. Integrating palliative care into comprehensive cancer care. J Natl Compr Canc Netw 2012;10:1192-8. [PubMed]
- Weeks JC, Catalano PJ, Cronin A, et al. Patients expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616-25. Available online: http://www.nejm.org/doi/full/10.1056/NEJMoa1204410 [PubMed]
- Teixeira AA, Hanvey L, Tayler C, et al. What do Canadians think of advanced care planning? Findings from an online opinion poll. BMJ Support Palliat Care 2015;5:40-7. [PubMed]
- Ipsos-Reid Survey. Hospice Palliative Care Study: Final Report, The GlaxoSmithKline Foundation and the Canadian Hospice Palliative Care Association, 2004:31.
- Ipsos Healthcare – The Healthcare Research Specialists, Canadian Hospice Palliative Care Association. Advance Care Planning Poll, 2012:7.
- Canadian Hospice Palliative Care Association. What Canadians Say: The Way Forward Survey Report December 2013. Ottawa (ON): Harris/Decima, 2013. Available online: http://www.hpcintegration.ca/media/51032/The%20Way%20Forward%20-%20What%20Canadians%20Say%20-%20Survey%20Report%20Final%20Dec%202013.pdf
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976;115:119-21. [PubMed]
- Hui D, De La Cruz M, Mori M, et al. Concepts and definitions for “supportive care,” “best supportive care,” “palliative care,” and “hospice care” in the published literature, dictionaries, and textbooks. Support Care Cancer 2013;21:659-85. [PubMed]
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009;115:2013-21. [PubMed]
- Canadian Cancer Research Alliance. Highlights in Investment in Palliative End-of-Life Care Cancer research in Canada, 2005-2010. Available online: http://www.ccra-acrc.ca/index.php/publications-en/investment-reports-special-topics/item/highlights-in-investment-in-palliative-and-end-of-life-care-cancer-research-in-canada-2005-2010
- Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011;29:1587-91. [PubMed]
- Debono DJ. Integration of palliative medicine into routine oncological care: what does the evidence show us? J Oncol Pract 2011;7:350-4. [PubMed]
- Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009;27:206-13. [PubMed]
- Rangachari D, Smith TJ. Integrating palliative care in oncology: the oncologist as a primary palliative care provider. Cancer J 2013;19:373-8. [PubMed]
- Canadian Hospice Palliative Care Association. Innovative Models of Integrated Hospice Palliative Care. The Way Forward Initiative: an Integrated Palliative Approach to Care. Ottawa (ON): The Association, 2013. Available online: http://www.hpcintegration.ca/media/40546/TWFinnovative-models-report-Eng-webfinal-2.pdf
- Fainsinger RL, Brenneis C, Fassbender K. Edmonton, Canada: a regional model of palliative care development. J Pain Symptom Manage 2007;33:634-9. [PubMed]
- Carlow DR. The British Columbia Cancer Agency: a comprehensive and integrated system of cancer control. Hosp Q 2000;3:31-45. [PubMed]
- Sisler JJ, DeCarolis M, Robinson D, et al. Family physicians who have focused practices in oncology: results of a national survey. Can Fam Physician 2013;59:e290-7. [PubMed]
- BC Cancer Agency. Family Practice Oncology Network. Available online: http://www.bccancer.bc.ca/healthprofessionals/networks/family-practice-oncology-network, accessed 1 May 2015.
- Bruera E, Michaud M, Vigano A, et al. Multidisciplinary symptom control clinic in a cancer center: a retrospective study. Support Care Cancer 2001;9:162-8. [PubMed]
- Watanabe SM, Fairchild A, Pituskin E, et al. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer 2013;21:1201-7. [PubMed]
- Gilbert JE, Howell D, King S, et al. Quality improvement in cancer symptom assessment and control: the Provincial Palliative Care Integration Project (PPCIP). J Pain Symptom Manage 2012;43:663-78. [PubMed]
- Dudgeon D, Knott C, Viola R, et al. Managing Continuity through Collaborative Care Plans: A Study of Palliative Care Patients. Kingston (ON): Canadian Health Services Research Foundation, 2004. Available online: http://www.cfhi-fcass.ca/Migrated/PDF/ResearchReports/OGC/dudgeon_final.pdf
- King S, Hughes E. Ontario’s Palliative Care Integration Project. Cancer Care Ontario, 2007. Available online: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13750
- Cancer Care Ontario. Regional Models of Care for Palliative Cancer Care: Recommendations for the Organization and Delivery of Palliative Cancer Care in Ontario. Provincial Palliative Care Program. The Association, 2009. Available online: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=77326
- Canadian Partnership Against Cancer. The 2012 Cancer System Performance Report. Toronto (ON): The Association, 2012. Available online: http://www.cancerview.ca/idc/groups/public/documents/webcontent/2012_system_performance_rep.pdf.
- Bryson J, Coe G, Swami N, et al. Administrative outcomes five years after opening an acute palliative care unit at a comprehensive cancer center. J Palliat Med 2010;13:559-65. [PubMed]
- Wentlandt K, Krzyzanowska MK, Swami N, et al. Referral practices of oncologists to specialized palliative care. J Clin Oncol 2012;30:4380-6. [PubMed]
- Chasen M, Bhargava R, MacDonald N. Rehabilitation for patients with advanced cancer. CMAJ 2014;186:1071-5. [PubMed]
- Gagnon B, Murphy J, Eades M, et al. A prospective evaluation of an interdisciplinary nutrition-rehabilitation program for patients with advanced cancer. Curr Oncol 2013;20:310-8. [PubMed]
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665-73. [PubMed]
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169:480-8. [PubMed]
- Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010;28:1203-8. [PubMed]
- Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program: a review of 2008- 2012. Curr Oncol 2013;20:e206-11. [PubMed]
- Danielson B, Fairchild A. Beyond palliative radiotherapy: a pilot multidisciplinary brain metastases clinic. Support Care Cancer 2012;20:773-81. [PubMed]
- Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer 2009;17:163-70. [PubMed]
- Lefresne S, Berthelet E, Cashman R, et al. The Vancouver rapid access clinic for palliative lung radiation, providing more than just rapid access. Support Care Cancer 2015;23:125-32. [PubMed]
- Wu JS, Kerba M, Wong RK, et al. Patterns of practice in palliative radiotherapy for painful bone metastases: impact of a regional rapid access clinic on access to care. Int J Radiat Oncol Biol Phys 2010;78:533-8. [PubMed]
- Pituskin E, Fairchild A, Dutka J, et al. Multidisciplinary team contributions within a dedicated outpatient palliative radiotherapy clinic: a prospective descriptive study. Int J Radiat Oncol Biol Phys 2010;78:527-32. [PubMed]
- Jung H, Sinnarajah A, Enns B, et al. Managing brain metastases patients with and without radiotherapy: initial lessons from a team-based consult service through a multidisciplinary integrated palliative oncology clinic. Support Care Cancer 2013;21:3379-86. [PubMed]
- Royal College of Physcians and Surgeons of Canada. Available online: http://www.royalcollege.ca/portal/page/portal/rc/public, accessed 1 May 2015.
- College and Association of Registered Nurses of Alberta. Available online: http://www.nurses.ab.ca/content/carna/home.html/, accessed 1 May 2015.
- Pallium Canada Interprofessional Education in palliative and end-of-life care. Available online: http://pallium.ca/, accessed 1 May 2015.


