Treatment of refractory gout with TNF-α antagonist etanercept combined with febuxostat
Introduction
Gout is a chronic rheumatic disease caused by hyperuricemia, resulting from the increase of purine biosynthesis or the disorder of uric acid excretion and causing the deposition of urate crystals synovium, bursa, cartilage, and other tissues. The diagnosis and treatment of complex and refractory gout is a major clinical problem to be solved. In the acute outbreak of gouty arthritis as a local acute inflammatory process, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and other cytokines play an essential role in the occurrence, development, and persistence of local inflammation in gout acute stage. Etanercept for injection is a recombinant human tumor necrosis factor receptor antibody fusion protein. One refractory gout was treated with TNF antagonists etanercept combined with febuxostat in our hospital, and satisfactory results were obtained. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2072).
Case presentation
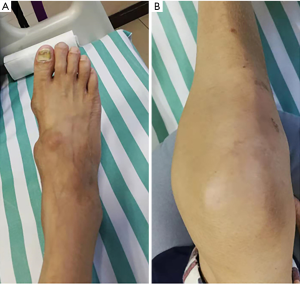
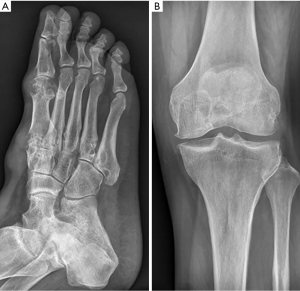
The patient was a 48-year-old male. He was admitted to the hospital on March 15, 2019, due to intermittent multiple joint swelling and pain for 17 years and aggravation for one month. He had hypertension and hyperlipidemia two years before this admission. Seventeen years ago, the patient developed pain of the first metatarsophalangeal joint of the right foot after drinking alcohol, accompanied by redness, swelling, and increased skin temperature. The disease was relieved one week after pain relief and symptomatic treatment at a local clinic. After that, swelling and pain of the metatarsophalangeal joints, ankle joints, knee joints, and hand joints of both sides occurred alternately, 5–6 times a year, and ibuprofen was given to relieve pain each time. Five years before this admission, the patient was hospitalized due to the reoccurrence of joint swelling and pain and received intravenous methylprednisolone in the hospital. After discharge from the hospital received continuous oral prednisone acetate 25 mg/day and intermittent analgesic treatment with ibuprofen. Although joint pain alleviated compared with before, swelling in bilateral knee joints gradually increased, and yellow-white nodules occurred in both hands and feet. One month before this admission, he suffered from swelling and pain of his right knee and right ankle joints, and ulceration with yellow-white secretion in his first metatarsophalangeal joint of his right foot after eating seafood. He was hospitalized in Jinan Central Hospital (Shandong Province, China). He was diagnosed as acute gouty arthritis, with uric acid 670 µmom/L. At the age of 52, he had the chalkstone removed from the first metatarsal toe of his right foot. Nausea accompanied by abdominal pain and discomfort under the xiphoid process occurred during the period of hospitalization. Gastroscopy showed mycotic esophagitis, reflux esophagitis, huge gastric ulcer (diameter about 2.5 cm), and superficial atrophic gastritis with erosion. The patient underwent febuxostat treatment 40 mg once a day, celecoxib 200 mg once a day, sodium bicarbonate 0.5 g t.i.d., rabeprazole 10 mg b.i.d. The symptoms of joint swelling and pain were not relieved. The wound was not healed after gout stone resection of the first metatarsophalangeal joint of the right foot. Yellow-white secretion could be observed after extrusion. The patient was discharged from the hospital due to poor treatment effects. For further diagnosis and treatment, the patient was admitted to our hospital due to swelling and pain of his right knee and ankle joints accompanied by fever. Physical examination: body temperature 38.8 °C, pulse 112 times/min, breathing 22 times/min, blood pressure 117/74 mmHg, looking like they were experiencing pain, swelling of interphalangeal joints of both hands with multiple yellow-white nodules (about 0.5–1 cm), and swelling, tenderness and limited movement of the right ankle joint and knee joints. Several yellow-white nodules with a size of 1–3 cm could be seen on the back of both feet (Figure 1). The scar after gout stone resection of the first metatarsophalangeal joint of the right foot could also be observed, and when it was extruded, yellow-white secretion was discharged. Blood routine examination: neutrophil absolute value, 17.02×109/L; white blood cell, 19.60×109/L; high sensitivity C-reactive protein, 208.7 mg/L and ESR: 84 mm/h (Table 1). Urine routine test: pH 6.5. Other examinations: fecal occult blood, weakly positive; antistreptolysin “O,” rheumatoid factor, tubercle bacillus antibody, negative; right foot secretion culture, positive Staphylococcus aureus; uric acid crystals are also observed in the secretion of the right foot under a polarized light microscope. Joint X-rays showed that the bone mineral density of both knee joints decreased slightly (Figure 2), the condylar eminence of the distal femur and the medial and lateral tibial intercondylar eminence became sharper, the articular surface became whiter, and the joint space was satisfactory. The suprapatellar bursas of both sides were inflamed. The bone density of both ankles was decreased, utricular lower-density shadows were observed under some articular surfaces, especially on the right side. The right ankle joint space was narrowed, and the left ankle joint space was acceptable. Gastroscopy showed mycotic esophagitis, mycotic phlebitis, gastric ulcer (about 2.3 cm in diameter), atrophic gastritis, and duodenitis. Urological ultrasonography showed there was no abnormality in the kidneys and ureters of both sides and the bladder.

Full table
Admission diagnosis: acute gouty arthritis (outbreak period), hypertension grade III, hyperlipidemia, mycotic esophagitis, inflammation of gastric cardia, gastric ulcer, atrophic gastritis, and duodenitis.
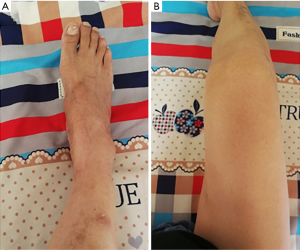
The treatment regimen administered was a low purine diet, alkalization of urine (to pH 6.2–6.9), omeprazole to inhibit gastric acid secretion, a small dose of colchicine to relieve joint pain, febuxostat to reduce uric acid synthesis, losartan potassium to reduce blood pressure, atorvastatin calcium tablets to lower lipid, and cefamandolate to resist infection. After the treatment above, the patient’s body temperature returned to normal, and his blood uric acid was 261 µmol/L, but joint swelling and pain occurred repeatedly. A selective cyclooxygenase (COX-2) inhibitor, a non-steroidal anti-inflammatory drug, celecoxib was added to treat the joint swelling and pain; however, multiple joint swelling and pain still did not relieve. In consideration of the ineffectiveness of celecoxib and colchicine in relieving the joint pain and the fungal infection of the upper gastrointestinal tract accompanied by a huge gastric ulcer, after tuberculosis, hepatitis, immunodeficiency disease, and tumor were excluded, 25 mg TNF antagonist etanercept was injected subcutaneously twice a week (72 hours interval) for two weeks. During this period, the uric acid lowering therapy with febuxostat was stopped. One day after the treatment with etanercept, the joint pain was relieved, the joint swelling disappeared in 3–5 days, the results of C-reaction protein (CRP) and blood routine examination returned to normal (Table 1), and the patient could stand on his own after two weeks. A treatment regimen administered after discharge from the hospital was febuxostat 40 mg once a day, colchicine 0.5 mg b.i.d. (the treatment maintained for half a year), sodium bicarbonate 0.5 g t.i.d., rabeprazole 10 mg b.i.d., losartan potassium 50 mg once a day, and atorvastatin 20 mg once a day. Follow-up visit: one month after the treatment with etanercept, the scar after gout stone resection of the first metatarsophalangeal joint of the right foot healed completely, joint swelling was reduced, the results of blood routine test and liver and kidney function tests became normal, and serum uric acid was 289 µmol/L. The telephone follow-up after hospital discharge told us that the condition was stable in 3 months, the gout stone disappeared in 6 months. One year later, gouty stones on the dorsum of both feet were significantly reduced (Figure 3), and gouty arthritis did not recur. Because the patient’s condition and clinical manifestations improved, considering economic factors and suspected factors, he refused to do X-ray examination. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
From the American College of Rheumatology (ACR) Guidelines to the European League Against Rheumatology (EULAR) Consensus, and then to the Chinese Gout Diagnosis and Treatment Guidelines and their many updated versions have given valuable guidance for the clinical diagnosis and treatment of gout. However, the disease background of patients involved in the guidelines and consensus is simple, and the treatment guidelines for complex and refractory gout is limited. The patient reported here had a disease course over ten years and had an irregular diet and nonstandard treatment. The disease is complicated with a fungal infection of the upper gastrointestinal tract and huge gastric ulcers. According to the latest guidelines, non-steroidal anti-inflammatory drugs (NSAIDs), colchicines, and glucocorticoids are the first-line drugs in treating acute gout attacks. Here, NSAIDs and glucocorticoids are contraindicated due to fungal infection of the upper gastrointestinal tract and huge gastric ulcer. When Staphylococcus aureus was positive in the culture of the secretion from the scar after gouty stone resection of the right foot, glucocorticoids were more unsuitable for the treatment because glucocorticoid applications might aggravate the infection. Therefore, we chose to add a relatively safe dose of colchicine (1 mg for the first time on the first day, and then 0.5 mg every 1–2 hours, a total dose in 24 hours not more than 6 mg, 1 mg from the second day, b.i.d., orally). The patient had been treated with febuxostat before admission as uric acid lowered treatment in the initial stage, but it was stopped due to the repeated attacks of joint pain. For uric acid inhibition and antifungal treatment, a selective inhibitor of COX-2, celecoxib (NSAIDs), was used to treat joint pain. The treatment continued for one week, but multiple joint swelling and pain still did not relieve. How to treat such acute, complex, and refractory gout? It has become a severe problem to be solved.
In the acute outbreak of gouty arthritis as a local acute inflammatory process, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and other cytokines play an essential role in the occurrence, development, and persistence of local inflammation in gout acute stage. Matsukawa et al. (1) detected the high expression of TNF-α and IL-1 mRNA in the experimental animal model of acute gouty arthritis and suggested that monosodium urate (MSU) crystal could directly stimulate monocytes in synovial fluid, leading to the production of TNF-α. TNF-α can enhance the activity of polymorphonuclear (PMN) elastase and increase the release of IL-1, which leads to an acute inflammatory reaction. Acute and chronic gouty arthritis is an inflammatory disease, in which activation of certain white blood cells occurs owing to the presence of a foreign substance—namely, urate crystals. The activation of monocytes and macrophages releases TNF-α into the synovial fluid, Increased concentrations of TNFα are detectable in joints of gouty arthriti. In certain cases, gout can mimic rheumatoid arthritis. In 2012, ACR guidelines for the treatment of gout mentioned that when the effect of conventional anti-inflammatory treatment is not satisfactory, the IL-1 blocker can be considered to control acute inflammation without gout indications. However, the drug has not yet been marketed in China; further studies have confirmed TNF-α participates in the pathogenesis of acute gouty arthritis. Chapman et al. (2) reported that the blockage of the production of TNF-α in gouty arthritis induced by MSU crystal could significantly inhibit the expression of E-selectin and the recruitment of PMN, and pointed out that TNF-α plays a vital role in the occurrence and development of gouty arthritis. Therefore, theoretically, TNF-α receptor antagonists can also be used as a part of a therapeutic regimen to control the acute development of gout.
Etanercept for injection is a recombinant human tumor necrosis factor receptor antibody fusion protein (rhTNF-α-FC), which binds with TNF-α in blood competitively, blocks its binding with TNF-α receptor on the cell surface, reduces its activity, and effectively controls arthritis (3). Etanercept alleviate the acute inflammatory response of gouty arthritis and achieve low uric acid treatment. It is still used off label for acute gout. However, several controlled studies in Europe, North America, and China have confirmed the efficacy and safety of etanercept in the treatment of rheumatoid and compulsive spondylitis, and the main clinical indications of the drug are rheumatoid arthritis and ankylosing spondylitis at present. Before the drug was used to the patient, we carried our joint consultation of the experts from the Pharmacy Department and Pharmaceutical Department of our hospital, got the approval of the Ethics Committee, Pharmacy Management, and Pharmacotherapeutics Committee, and got informed consent of the patient and his family after having explained the possible adverse reactions and complications of the drug.
The patient responded well to etanercept, and the joint pain was significantly relieved one day after treatment, and entirely relieved after five days. Two weeks later, the results of C-reaction protein (CRP) and blood routine examination returned to normal. It is suggested that TNF antagonists can rapidly relieve acute gouty arthritis. Also, the fact that during the treatment, the patient repeatedly suffered from joint swelling and pain, which could not be relieved with colchicine and NSAIDs, suggests the involvement of uric acid lowering drugs cannot be excluded. It is well-known that the acute attack of gout is related to the fluctuation of uric acid caused by the dissolution of gouty stone. The initiation of uric acid-lowering therapy can lead to repeated occurrence of gouty arthritis, especially 2–3 months after the beginning of treatment. Although the uric acid lowering drugs can significantly reduce the level of blood uric acid, the dissolution and fluctuation of uric acid caused by the drugs can cause repeated attacks of acute gouty arthritis. When to restart uric acid-lowering therapy? ACR guidelines 2012 suggest that the uric acid lowering therapy can be started after the effective anti-inflammatory treatment is started. However, EULAR Consensus 2013 mentions that the relevant studies have not reached a consistent conclusion. Chinese Gout Diagnosis and Treatment Guidelines suggest the uric acid lowering treatment should be started at least two weeks after the acute gout remission. The application of TNF antagonists effectively alleviated joint pain and supplied an opportunity for patients to start uric acid-lowering therapy. Uric acid-lowering therapy with febuxostat was restarted after two weeks of pain relief, here, considering the heavy uric acid load of the patient and multiple gout stones.
Tausche et al. (4) reported that etanercept successfully treated one case of refractory gout in 2004, and the proposed etanercept was a new choice for refractory gout patients with low effect on common anti-inflammatory drugs. However, probenecid, a uric acid excretion drug used in the treatment, could only reduce the level of serum uric acid to 560 µmol/L and rarely to the target value below 300 µmol/L, although the acute attack of gouty arthritis was reduced. Han et al. (5) and Shi et al. (6) reported one case observation of refractory gout treated with etanercept in China. Han et al. (5) chose allopurinol 0.1 g once a day after the acute gout stage, while Shi et al. (6) chose etanercept combined with febuxostat 40 mg once a day to reduce uric acid below the target level of 300 µmol/L. They all supported stable patient conditions and achieved the shrinkage and disappearance of gout stones. For uric acid lowering treatment, the target value of blood uric acid is proposed in most gout therapy guidelines. The consensus is through the characteristic that many urate crystals will be precipitated when uric acid is over 420 µmol/L in a purely physical and chemical environment. Therefore, the target value of serum uric acid in most gout therapy guidelines is 360 µmol/L. In 2017, the British Society for Rheumatology (BSR) guidelines even proposed a target value of 300 µmol/L. It is believed that the lower level of uric acid is helpful to dissolve gouty stone, but the specific mechanism is still unclear, which may be related to various inflammatory cytokines. TNF antagonists not only alleviate the acute inflammatory response of gouty arthritis but also ensure the implementation of uric acid-lowering therapy. For patients, TNF antagonists are expensive, so it is still vital to give a low purine diet. Simultaneously, it is necessary to be alert to infection, tumor, and other related adverse reactions in using this drug. The safety and efficacy of the drug in the treatment of acute, complex, and refractory gout still need to be confirmed by randomized, multi-center, and extensive sample controlled clinical studies. The patients also expressed their deep gratitude to our team. This case report only supplies a new reference scheme for the treatment of similar diseases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2072
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2072). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Matsukawa A, Yoshimura T, Maeda T, et al. Analysis of the cytokine network among tumor necrosis factor alpha, interleukin-1beta, interleukin-8, and interleukin-1 receptor antagonist in monosodium urate crystal-induced rabbit arthritis. Lab Invest 1998;78:559-69. [PubMed]
- Chapman PT, Yarwood H, Harrison AA, et al. Endothelial activation in monosodium urate monohydrate crystal-induced inflammation: in vitro and in vivo studies on the roles of tumor necrosis factor alpha and interleukin-1. Arthritis Rheum 1997;40:955-65. [Crossref] [PubMed]
- Huang F, Zhang J, Zheng Y, et al. A multicenter, double-blind, randomized, placebo-controlled clinical trial of etanercept treatment of Chinese patients with active ankylosing spondylitis. Zhonghua Nei Ke Za Zhi 2011;50:1043-7. [PubMed]
- Tausche AK, Richter K, Grässler A, et al. Severe gouty arthritis refractory to anti-inflammatory drugs: treatment with anti-tumour necrosis factor alpha as a new therapeutic option. Ann Rheum Dis 2004;63:1351-2. [Crossref] [PubMed]
- Han Y, Qiu H, Yang W, et al. Successful treatment of refractory gout by TNF antagonist, one case report and literature review. Tianjin Medical Journal 2013;41:181-2.
- Shi L, Xu J, Gao H, et al. Successful treatment of refractory gout by febuxostat combined with etanercept: One case report. Chinese Journal of Endocrinology and Metabolism 2017;33:522-4.
(English Language Editor: J. Chapnick)