
Nivolumab in combination with anlotinib achieved remarkable efficacy in a patient with driver-negative lung squamous cell carcinoma and PS of 4
Introduction
Both morbidity and mortality of lung cancer ranks first among all cancer types in China, of which 85% are patients with non-small cell lung cancer (NSCLC) (1). Patients with NSCLC and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 3–4 do not tolerate chemotherapy well, and these patients are underrepresented in clinical trials. Standard treatment is generally recommended in patients with PS of 0–1, while best supportive care is offered to patients with PS of 3 or greater.
There is difference in the efficacy of different immune checkpoint inhibitors due to various drug structure, tumor types and pathological types. Nivolumab is a human IgG4 PD-1 antibody that can restore anti-tumor immunity by targeting PD-1 mediated signaling (2). Both the CheckMate 017 trial and the CheckMate 057 trial showed that nivolumab has better efficacy and safety than chemotherapy (3,4). Anlotinib is a small molecule multitarget tyrosine kinase inhibitor (TKI) targeting VEGFR, PDGFR, FGFR, and c-Kit, which has been shown to inhibit tumor growth and angiogenesis (5). A phase III trial showed that anlotinib significantly prolonged patients’ survival time regardless of EGFR mutation or pathological types compared to placebo, with a tolerable toxicity profile (6). However, there is a complete lack of reports on the effects of these treatments on driver-negative NSCLC patients with PS of 4.
Here we reported a driver-negative lung squamous cell carcinoma patient with PS of 4 who was successfully treated with nivolumab combined with anlotinib. Our case report showed that immunotherapy combined with antiangiogenesis was a good choice for advanced driver-negative lung squamous cell carcinoma patient with PS 4. We present the following case report in accordance with the CARE guidelines (available at http://dx.doi.org/10.21037/apm-20-2096) (7).
Case presentation
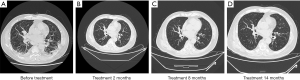
A 72-year-old man presented to the ICU of our hospital for cough and dyspnea in January 2019. He was diagnosed with type 1 respiratory failure, acute exacerbation of chronic bronchitis, and acute kidney injury, accompanied by comorbidities such as grade 3 hypertension, diabetes, and coronary heart disease. He received treatment of oxygen uptake, anti-infection, resolving sputum and relieving asthma in the local hospital, but his symptoms didn’t improve. Chest computed tomography (CT) scans revealed enlarged lymph nodes in the right hilum and mediastinum (Figure 1A). His emission computed tomography (ECT) is normal and brain magnetic resonance imaging (MRI) showed no brain metastasis. His Acute Physiology and Chronic Health Evaluation (APACHE-II) score was 18, and the risk factor for death was 23.56%. After 1 week, the patient was out of danger. Both trans-bronchial biopsy and CT-guided biopsy confirmed stage IV lung squamous cell carcinoma, and gene sequencing revealed that EGFR, ALK, ROS1, MET, and BRAF genes were wild type. Due to his PS of 4 and comorbidities, we decided to use the best supportive treatment (BSC), but the patient and his family strongly sought anti-tumor treatment. Considering that immunotherapy and anti-angiogenic therapy are generally better tolerated than conventional chemotherapy in these patients, after informing the patient of the risk-benefit ratio of treatment and obtaining informed consent, we tried administering nivolumab (200 mg on day 1 of each 21-day cycle) in combination with anlotinib (12 mg per day for 2 weeks, every 3 weeks) treatment to the patient from January 31, 2019. If patient has tumor progression or intolerant adverse event, the combination will be interrupted. We didn’t test patient’s PD-L1 expression level because the efficacy of immunotherapy for patients with different PD-L1 expression levels still remain controversy. After 2 months of combined treatment, we observed an improvement in PS and according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version1.1), local lesions were partial response (PR) (Figure 1B). At the same time, patient’s symptoms has relieved, such as lower frequency of dyspnea and cough. The patient has continued to be supervised with biochemical indicators values and serum tumor marker measurements, founded no bad signs associated with the treatment. Re-examination over the longer term showed that the tumors remained stable and no adverse events occurred during the treatment period (Figure 1C,D). At present, the patient is still being followed-up with a progression-free survival (PFS) of 14.0 months.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
To our knowledge, this is the first report demonstrating the efficacy of nivolumab combined with anlotinib for the treatment of a driver-negative lung squamous cell carcinoma patient with PS of 4. Our findings provide a novel treatment approach for patients with poor PS.
A large proportion of lung cancer patients will have concurrent diseases or comorbidities, which may affect their ability to receive standard lung cancer treatment. In our case, the patient had acute exacerbation of chronic bronchitis and several comorbidities, resulting in a PS of 4. Individuals with poor or critical PS account for a large proportion of all lung cancer patients. Studies show that patients with an ECOG PS of 2 account for 30–40% of all patients with NSCLC (8). PS is a predictive factor for toxicity and cancer response. Current evidence indicates that regardless of age, the incidence of adverse effects from standard chemotherapy increases in lung cancer patients with poor PS, and the overall survival rate (OS) is poor (9). Unfortunately, due to the stringent inclusion criteria, these individuals are underrepresented in conventional clinical trials, leading to a lack of evidence for the use of other new treatment regimens in these patients.
Research has shown that single-agent erlotinib should not be used instead of chemotherapy for unselected patients in the first-line setting in the absence of known EGFR mutations. Targeted drugs should be provided to patients according to the mutation status regardless of their PS (10,11). The Trial of Poor Performance Status Patients (ToPPS) assessed the efficacy of bevacizumab in the first-line treatment of PS 2 patients with NSCLC. In this study, despite the improved response rates and comparable toxicity, no survival benefit was observed in patients treated with bevacizumab (12). Therefore, there is currently no evidence to support the use of angiogenic inhibitors in PS 2 patients. The CheckMate 153 and CheckMate 171 trials found the median OS was only 3.9 months and 5.4 months, respectively, in patients with PS 2 treated with nivolumab (13,14). Some retrospective studies have reported that the efficacy of immune checkpoint inhibitors (ICIs) for advanced NSCLC patients with PS ≥2 was poor regardless of PD-L1 expression, and response rates were 5–20% with a median PFS of 0.8–2.4 months (15-17). In our case, an angiogenic inhibitor combined with an ICI achieved PFS ≥14 months in a patient with PS of 4, resulting in a greater efficacy compared to the above studies.
Immune checkpoint inhibitors monotherapy has been standard and preferential therapeutic strategy in NSCLC showing better survival benefit, but there is also the problem of drug resistance and serious adverse event. Anlotinib as a novel multitarget antiangiogenesis, has been proved as an effective posterior line treatment in advanced non-small cell lung cancer. Anlotinib provide a potential for patients who unable to benefit from chemotherapy, immunotherapy and EGFR-mutation target agent (6). In the IMpower150 and TASUKI-52 trials, ICIs combined with angiogenic inhibitors and chemotherapy showed a significant PFS benefit in NSCLC patients compared to the combination of angiogenic inhibitors and chemotherapy (18,19). At the World Conference on Lung Cancer (WCLC) in 2019, a phase 1 trial with sintilimab (ICI) plus anlotinib as first-line treatment in advanced NSCLC patients without oncogenic driver mutations showed encouraging results (20). These studies suggest that the anti-angiogenic drug has an immunomodulatory effect and can enhance immune efficacy.
Meanwhile, safety of combination therapy also merit consideration. In the TASUKI-52 trial, no new signs of adverse reactions was found. Another single-arm phase 2 clinical trial with atelizumab plus bevacizumab enrolled 40 advanced NSCLC patients, reported 23.1% of atelizumab plus bevacizumab related adverse event (SAE) and the adverse effects rate is acceptable (21). In this case report, our patient has not happened immunotherapy-related adverse events (irAEs). IrAEs caused by immune checkpoint inhibitors having effects on other normal tissues, disrupt the balance between tolerance and immunity (22).The serious irAEs which grade ≥3 may associated with long-term effect of immune checkpoint inhibitors on other normal tissues. It should be noted that irAEs can present at any time, monitoring the adverse reactions in the follow-up course is necessary. We have followed he NCCN Guidelines for Management of Immunotherapy-Related Toxicities (Version 1.2020) to monitor the patient during the treatment.
In summary, our successful treatment of this driver-negative lung squamous cell carcinoma patient provides valuable real-word evidence to synergistic effect of immunotherapy and anti-angiogenesis therapy, and also demonstrated the potential application of this treatment regimen in critically ill patients. Further studies in more cases will be required to confirm the efficacy and safety of immunotherapy combined with anlotinib for NSCLC and clinical trials will be thus warranted to confirm our results in the future.
The patient has given satisfaction for the efficacy of the treatment. He is willing to continue this treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist Available at http://dx.doi.org/10.21037/apm-20-2096
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2096).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846-56. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77-86. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Lilenbaum R, Cashy J, Hensing T A, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [Crossref] [PubMed]
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001;92:2639-47. [Crossref] [PubMed]
- Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9. [Crossref] [PubMed]
- Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253-60. [Crossref] [PubMed]
- Lilenbaum R, Hainsworth J, Joseph M, et al. Randomized phase II study of pemetrexed v. pemetrexed/bevacizumab v. carboplatin/pemetrexed/bevacizumab in patients with previously untreated advanced non-squamous non-small-cell lung cancer and poor performance status. J Thorac Oncol 2013;8:S889-90.
- Spigel D R, Schwartzberg L S, Waterhouse D, et al. Is Nivolumab Safe and Effective in Elderly and PS2 Patients with Non-Small Cell Lung Cancer (NSCLC)? Results of CheckMate 153. J Thorac Oncol 2017;12:S1287-8. [Crossref]
- Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer 2020;127:160-72. [Crossref] [PubMed]
- Facchinetti F, Mazzaschi G, Barbieri F, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer 2020;130:155-67. [Crossref] [PubMed]
- Watanabe S, Goto Y, Motoi N, et al. Nivolumab in Elderly or Poor Performance Status Patients with Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:S1338-9. [Crossref]
- Koyama J, Kimura T, Oi H, et al. Immune checkpoint inhibitor for advanced or recurrent non-small cell lung cancer patients with poor performance status. Ann Oncol 2019;30:vi141. [Crossref]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Lee JS. Randomized phase III trial of nivolumab in combination with carboplatin, paclitaxel, and bevacizumab as first-line treatment for patients with advanced or recurrent non-squamous NSCLC. ESMO 2020,LBA#54.
- Han B, Chu T, Zhong R, et al. Efficacy and Safety of Sintilimab with Anlotinib as First-Line Therapy for Advanced Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2019;14:S129. [Crossref]
- Seto T. WJOG @Be study: A phase II study of atezolizumab (atez) with bevacizumab (bev) for non-squamous (sq) non-small cell lung cancer (NSCLC) with high PD-L1 expression. ESMO 2020,LBA#55.
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-68. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


