
The clinical value of multimodal ultrasound for the evaluation of disease activity and complications in inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a group of non-specific inflammatory diseases of the digestive tract. The main types of IBD are Crohn’s disease (CD) and ulcerative colitis (UC). IBD is typically characterized by repeated relapse and remission after effective clinical treatment. Patients need to undergo multiple follow-up examinations and evaluations throughout their lives. IBD affects patients’ physical and mental health and quality of life (1).
Early diagnosis of IBD and effective evaluation of inflammatory activity and complications can help guide clinical treatment, including drugs and timely surgical intervention, which can benefit patients greatly.
Endoscopy is an important means of clinically evaluating IBD. The diagnosis and evaluation of IBD is achieved through the visualization of microscopic mucosal healing, which has been established as one of the key treatment goals for patients with the disease (2). However, CD is well known to be a transmural inflammation, endoscopic mucosal healing does not mean full-thickness healing of the intestinal wall, and can involve mesenteric fat and lymph nodes. It is often accompanied by penetrating complications, such as fistulas and abscesses which cannot be detected by colonoscopy, meaning the scope and severity of the disease cannot be comprehensively assessed. Moreover, colonoscopy is an invasive procedure, and some patients have poor tolerance and a risk of perforation.
Currently, magnetic resonance imaging (MRI) and computed tomography (CT) are the most commonly used imaging tools for assessing intestinal diseases. They have high accuracy for the diagnosis of IBD, and for monitoring and evaluating confirmed cases (3). However, CT carries the risk of radiation exposure, MR is time-consuming and expensive, and the contrast agents used for both imaging techniques are nephrotoxic. Therefore, new, non-invasive, and effective imaging methods have been explored. Ordas et al. (4), for instance, summarized the advantages and limitations of endoscopy and cross-sectional imaging techniques including ultrasound, CT, and MRI in assessing the course of IBD.
Gastrointestinal ultrasound is more easily accepted by patients because it is non-invasive and inexpensive, and has non-ionizing radiation. Its importance in the clinical diagnosis and treatment of IBD is also increasing. Previous studies have focused on the clinical application of single-mode ultrasound, such as two-dimensional and Doppler ultrasound. However, with the continuous development of ultrasound technology, new technologies, including intravenous contrast-enhanced ultrasound (CEUS) and elastic ultrasound, have continued to emerge. The combined application of multiple ultrasound technologies will allow for more comprehensive and effective evaluation of IBD, which will improve clinical management strategies.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2162).
Methods
Research object
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical committee of Tianjin Medical University General Hospital (No. IRB2020-WZ-163). Inpatients with CD and UC who were admitted to Tianjin Medical University General Hospital between September 2017 and December 2019 were enrolled in this study. All included patients met the diagnostic criteria of the third European evidence-based consensus diagnosis and management of Crohn’s disease and ulcerative colitis 2016 (5,6). The exclusion criteria were: aged <18 years; pregnant women; and recent acute or chronic heart failure.
This study was conducted according to the relevant medical ethics regulations, and all research subjects signed the informed consent form. The Crohn’s Disease Activity Index (CDAI) and the Mayo scoring system were adopted to assess disease activity, and laboratory data including the levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were collected. The interval between the measurement of all clinical and laboratory data, and the auxiliary examinations of patients was no longer than 2 weeks, and the patients’ treatment plans remained unchanged during this period.
Ultrasound
Two-dimensional ultrasound
Before undergoing ultrasound, patients fasted for 6–8 hours without special bowel preparation. The patients were examined with the LOGIQ E8 ultrasound instrument from American GE Corporation. First, a low-frequency convex array probe C1-5 was used to scan the whole abdomen counterclockwise from the sigmoid colon, and long- and short-axis scans were performed separately. After the positive area was found, a high-frequency linear array probe ML6-15 was used for further inspection.
Observation indicators included measurement of the full thickness of the intestinal wall on the cross section of the intestinal wall (the distance from the hyperechoic line between the intestinal cavity and the mucosa to the hyperechoic line between the muscularis propria and the serosa). If multiple intestinal segments were involved, then the maximum wall thickness was taken as the final result. Other indicators included the extent of lesion involvement, intestinal wall stratification, intestinal motility, intestinal stenosis, fistula, abscess, mesenteric fat wrap, and mesenteric lymph node enlargement. Mesenteric fat thickening manifests as a hyperechoic area surrounding the diseased intestine, and abnormally enlarged lymph nodes manifest as an anechoic or hypoechoic area exceeding 5 mm in diameter.
Doppler ultrasound
A high-frequency linear array probe ML6-15 was used to adjust the area of the color sampling frame at the thickest part of the bowel wall to reduce flicker artifacts. The energy gain was adjusted so that the sampling frame was completely covered by the color, and then gradually decreased until only the intravascular signal could be seen. The blood flow signal was evaluated using the Limberg score: grade 0: normal intestinal wall; grade 1: thickened intestinal wall with no blood flow signal; grade 2: thickened intestinal wall with shorter blood vessels; grade 3: thickened intestinal wall with long blood vessels; grade 4: thickened intestinal wall with long blood vessels connected to the mesentery (7).
CEUS
A low-frequency convex array probe C1-5 was used to detect the thickest part of the intestinal wall (for patients with a history of surgery, the thickness was measured at least 1 cm away from the anastomosis to prevent the suture from being detected and influencing the measurement result). The technical parameters were adjusted to wideband harmonic imaging mode, low mechanical index 0.06–0.08, dynamic range 65 db, and frame rate 10–13 frames/s. The patient was instructed to breathe calmly. Then, the contrast agent SonoVue (Bracco, Suisse SA) 2.4 mL was injected through the anterior elbow vein intravenously, and the tube was flushed with 5 mL 0.9% NaCl. Following the administration of the contrast agent, images were recorded for 90 s, then collected and stored in video format. The sampling frame for the region of interest (ROI) was selected to include the thickened intestinal wall and exclude the intestinal cavity and mesentery, and the abdominal muscles were selected as a control. The ultrasonic instrument’s time intensity curve (TIC) analysis system was used to obtain the TIC, and dynamic quantitative parameters, including time to peak (TTP), peak intensity (PE), and the area under the curve (AUC).
Elastic ultrasound
A high-frequency 9 L linear array probe was used to detect the thickest part of the intestinal wall. The gray-scale image mode was switched to the shear wave elastic ultrasound mode, and t and set the imaging condition to display the shear wave velocity and Young’s modulus automatically when the imaging condition was not pressurized. The probe is perpendicular to the body surface, without pressure, ask the patient to hold their breath and press the elastic imaging button. The probe was allowed to stand for 6 seconds. After the image was stable, it was saved at a fixed frame, and the ROI was selected. Region of interest (ROI) select 3 o’clock or 9 o’clock position for axial placement, with shear wave propagation velocity (m/s) and Young’s modulus value (kPa) as the evaluation indexes. Images were collected three times repeatedly. The Young’s modulus value and shear wave velocity automatically measured by the system were recorded, and the average value was taken.
Colonoscopy
The patient needs to fast for more than 6 hours before the colonoscopy. After taking 2,000 mL polyethylene glycol electrolyte solution to clean the intestines, perform colonoscopy (Olympus, probe CF 260, Japan), and routinely take biopsy for histopathological evaluation (8,9). UC and CD were assessed using the Mayo endoscopic score (MES) and simplified endoscopic score for Crohn’s disease (SES-CD) (the Rutgeerts score was used for patients after CD surgery). The results of colonoscopy were taken as the reference standard.
Whole-abdomen enhanced CT
The patient needs to fast for more than 6 hours before the procedure. Polyethylene glycol electrolyte solution (2,000 mL) was taken to clean the intestines, and the patients were placed in the supine position. CT was performed using an ICT scanner from Philips in the Netherlands, with the scanning parameters set as follows: 300–600 mA, 120 kvp, slice thickness 1.0 mm, pitch 1.375, FOV 45 cm, matrix 512×512. Contrast agent (Iohexol 80 mL) was injected intravenously at an average rate of 3 mL/s. The CT images were analyzed separately by two experienced abdominal radiologists, who were blinded to the clinical, endoscopic, and laboratory data. In cases of disagreement between the two radiologists, a consensus was reached through discussion. Positive results included wall thickness, stratification, extent of the lesion, location of the lesion, enlarged lymph nodes, increased mesenteric fat density, and pectinate. Complications included stenosis, fistula, and abscess.
Statistical analysis
Statistical analyses were performed with SPSS 25.0 statistical software. The statistical description of measurement data is expressed by the mean ± standard deviation, and the data comparison uses the t test and the Mann-Whitney U test. The statistical description of the count data is expressed by frequency, and the data comparison uses Fisher’s exact test. Correlation analysis uses Spearman rank and Pearson correlation analysis, and P<0.05 is considered statistically significant.
Results
Patient clinical data
Thirty patients with IBD were included retrospectively, including 20 males and 10 females. The patients were aged 20–70 years old, and the average age for the entire cohort was 43.37±16.99 years old. There were 15 patients with CD, with an average age of 36.87±16.14 years old, and 15 patients with UC, with an average age of 49.87±15.72 years old. With colonoscopy used as a reference standard, the patients were divided into the remission group and disease activity group. There were 10 cases (5 males and 5 females) in the remission group, and the average age was 41.1±15.0 years old. The active group comprised 20 cases (15 males and 5 females) with an average age of 44.50±18.17 years old. There was no statistically significant difference in age or sex distribution between the two groups, and the data were comparable. Three patients with CD had undergone bowel resection due to bowel perforation or obstruction. The patients’ clinical data are shown in Table 1. Clinical scores were collected on the day of the ultrasound examination, and the interval between laboratory examination collection and ultrasound examination was less than 1 week, and the interval between ultrasound, colonoscopy, and CT examination time were all completed within 2 weeks.
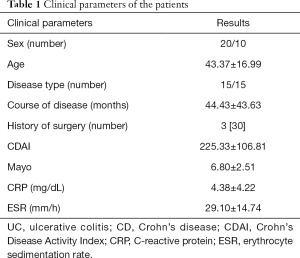
Full table
IBD appearance on ultrasound
All patients successfully underwent multimodal ultrasound examinations, and no obvious adverse reactions occurred. A total of 24 patients underwent quantitative analysis using CEUS, and patients were excluded due to obvious intestinal peristalsis, which affected the results. All ultrasound examinations were completed by an same experienced sonographer, who was unaware of the patient’s clinical data and auxiliary examination results.
Ultrasonography revealed that the lesions of 15 UC patients were located in the colon and rectum. The lesions of CD patients are diverse, of which 5 patients are located in the ileum and cecum, 5 patients are located in the colon and rectum, and 5 patients are located in the ileum and colon. In the entire cohort, 8 patients had single bowel disease and 22 patients had multiple bowel disease. The average maximum thickness of all diseased bowel walls was 6.67±2.87 mm. Among the CD patients, there were 10 cases of mesenteric fat thickening and 7 cases of abnormally enlarged mesenteric lymph nodes. In the patients with UC, there were 5 cases of mesenteric fat thickening in patients with UC and 4 cases of abnormal mesenteric lymph nodes, all patients in the cohort had active disease. Twenty-one patients had a Limberg score of grade 2–4, and 3 patients were in remission, and the Limberg scores of these 3 patients were all at level 2. The average elastic ultrasonic shear wave velocity was 2.77±0.86 m/s, and the average Young’s modulus was 26.85±13.96 kPa. In terms of the CEUS quantitative parameters, the mean PE was −43.93±6.71 dB, the mean TTP was 20.06±9.36 sec, and the mean AUC was 302.43±166.33 dBsec.
Ultrasound assessment of IBD activity
With colonoscopy taken as the reference standard, there were 20 patients in the active group and 10 patients in the remission group. Intestinal wall thickness was thicker in the active group than in the remission group, and the difference was significant (7.93±2.65 vs. 4.16±1.08 mm, P<0.001). In the active disease group, 18 cases had color Doppler score (Limberg score) ≥2 and 2 cases were <2; in the remission group, there were 3 cases with Limberg score ≥2 and 7 cases were <2. The score difference between the two groups was statistically significant (P=0.002). In the active group, there were 9 cases with clear intestinal wall stratification and 11 cases with unclear intestinal wall stratification, compared with 8 cases and 2 cases, respectively, in the remission group. The difference was not statistically significant (P>0.05).
The CEUS perfusion mode showed that the direction of contrast agent perfusion was from the serosal layer to the mucosal layer. In terms of CEUS parameters, the PE and AUC values in the active group were higher than those in the remission group, and the difference was significant (−40.66±4.81 vs. −50.47±5.03 db, P<0.001; 356.44±170.67 vs. 194.42±92.09 dBsec, P=0.021); however, the difference in TTP values between the active group and remission groups of patients was not statistically significant (20.04±8.74 vs. 20.09±11.13 s, P=0.990). ROC curve analysis showed that, with an intestinal wall thickness of >4.7 mm set as the cut-off value, the sensitivity diagnosing IBD disease activity was 90% and the specificity was 80%, with an AUC of 0.885. When the cut-off value for PE, TTP, and AUC were set as 44.37 dB, 10.73 s, and 226.15 dBsec, respectively, the diagnostic sensitivity was 75%, 94%, and 81%, and the specificity was 100%, 25%, and 75%, respectively. The corresponding AUC values were 0.922, 0.516, and 0.824. The performance of ultrasound parameter assessment activity is shown in Table 2.

Full table
Ultrasound evaluation of IBD complications
Twenty cases of intestinal stenosis were detected by ultrasound (using colonoscopy as the diagnostic criteria, 20/20). No fistulas or abscesses were detected (using CT as the diagnostic criteria). CEUS and elastic ultrasound were used to identify the cause of intestinal stenosis (inflammatory stenosis vs. fibrotic stenosis): according to the stenosis score standard (10), the patients were divided into two groups: Fibrosis group and inflammation group. The shear wave velocity (3.63±0.86 vs. 2.51±0.66 m/s, P=0.004), Young’s modulus (42.11±14.52 vs. 21.41±8.78 kPa, P=0.001), and TTP value (30.08±6.60 vs. 14.39±3.18 s, P<0.001), PE value (−46.84±7.65 vs. −40.41±5.10 dB, P=0.036), and AUC value (204.38±42.53 vs. 417.95±160.15 dBsec, P=0.001) of the two patient groups were significantly different (Table 3). Stenosis score was found to be significantly negatively correlated with shear wave velocity, Young’s modulus, and TTP, and the respective r values were −0.593, −0.662, and −0.754 (all P<0.05). Stenosis score was found to have a significant positive correlation with PE and AUC, and the r values were 0.450 and 0.643, respectively (both P<0.05).

Full table
Correlation analysis
In the UC group, intestinal wall thickness was strongly positively correlated with endoscopic score (r=0.753, P<0.05), and the Doppler classification showed a moderate positive correlation with endoscopic score (r=0.546, P<0.05). The quantitative parameters PE, AUC and endoscopy score have a strong positive correlation (r=0.757, 0.693, p<0.05), and there is no significant correlation between TTP and endoscopy score (p>0.05). In the CD group, Intestinal wall thickness was strongly positively correlated with endoscopic score (r=0.678, P<0.05), and the Doppler grading showed a strong positive correlation with endoscopic score (r=0.710, P<0.05), PE and AUC show a moderately strong positive correlation with endoscopy score (r=0.566 and 0.571, P<0.05), and no significant correlation was found between TTP and endoscopy score (P>0.05). Ultrasound and CT revealed a significant positive correlation between the maximum wall thickness of the diseased bowel (r=0.799 and 0.831, P<0.05) and the extent of the disease. Intestinal wall thickness all showed a moderately strong positive correlation with CRP and ESR (r=0.48 and 0.432, P<0.05), while intestinal wall thickness and Mayo score were strongly positively correlated (r=0.703, P<0.05). However, no significant correlation was found between intestinal wall thickness and CDAI (P>0.05).
Discussion
IBD is characterized by a course of repeated chronic relapse and remission. Endoscopy is an important method used for the clinical diagnosis and evaluation of IBD. However, as an invasive procedure, some patients have poor tolerance and a risk of perforation. MR and CT are the most commonly used imaging assessment tools. MR and CT are the most commonly used imaging evaluation tools, but CT has radiation exposure risks, and MR is time-consuming and expensive. Ultrasound is non-invasive and affordable, and does not expose patients to radiation; these qualities have seen it play an increasingly important role in the diagnosis and treatment of IBD. Compared with other cross-sectional imaging methods, ultrasound can be used to dynamically observe intestinal peristalsis and intestinal wall compressibility in real time, quickly measure intestinal wall thickness, observe intestinal wall stratification, and clarify the location and length of the diseased intestinal segment and the involvement of the mesentery. It can also be used to obtain other information about intestinal complications, such as abscesses, fistulas, and stenosis (11). A meta-analysis showed that ultrasound, CT, and MRA are highly accurate in identifying fistulas, abscesses, and stenosis in CD patients (12). CT is widely used in the diagnosis and evaluation of IBD in clinical practice. E. Calabrese’s study found a strong correlation between contrast ultrasound and CT in measuring intestinal wall thickness and lesion range (13).
Thickening of the bowel wall is the most prominent and most common ultrasound manifestation in patients with IBD during the active phase (14), and has good repeatability (15). Studies have shown that the most effective index for predicting endoscopic remission after clinical treatment is bowel wall thickness (16). Through correlation analysis, this study found that endoscopic activity score has a good correlation with intestinal wall thickening measured by two-dimensional ultrasound, color Doppler, and CEUS. Among ultrasound parameters, two-dimensional ultrasound measurement of intestinal wall thickening shows high sensitivity, specificity and accuracy in diagnosing disease activity. The hemodynamic parameters used in spectral Doppler blood flow signal analysis, such as the resistance index, are quite different in diagnosing disease activity (17,18). In this study, the Limberg score was used to semi-quantitatively analyze the blood flow signal, which was compared with the endoscopic score. The accuracy of color Doppler is still limited in the detection of low-velocity blood flow in small blood vessels in the bowel wall and blood flow in the deep bowel. In recent years, an increasing number of studies have shown that intestinal wall perfusion is an indicator of disease activity, while CEUS can improve the accuracy of detecting tiny blood vessels. Giangregorio et al. proposed that the increase in intestinal wall perfusion as a measurable parameter should be an indicator of inflammatory activity rather than the presence of vascularization and contrast enhancement (19). Some studies have focused on the dynamic parameters of CEUS. Although there is no agreement on which TIC parameter is more important in the assessment of disease activity, the overall trend is that volume parameters (PE and AUC) are positively correlated with reference standards, while flow parameters (TTP, Rise time, and Mean transit time) are negatively correlated (20,21). In this study, endoscopy score was positively correlated with the volume parameters PE and AUC, but had no significant correlation with the flow parameter TTP. CEUS analysis showed that the TTP value of the remission group was shorter than that of the active group, which may be related to the controversial reliability of dynamic perfusion analysis when the intestinal wall is thinner (22), and may also explain the lack of significant difference in TTP between the two groups. However, the underlying reason for this needs to be confirmed in future studies. The qualitative analysis of CEUS in this study found that the direction of perfusion was from the serosal layer to the mucosal layer. In his study, Migaleddu et al. proposed that the perfusion of the contrast agent from the submucosa to the serosal layer suggests active inflammation. (23), which is inconsistent with the results of this study. The reason for this inconsistency is that qualitative analysis based on visual analysis is often subjective, which leads to greater differences in results. Echo enhancement of the submucosa is related to fibrosis, while the hypoechoic changes of intestinal wall stratification are related to inflammatory edema and vascularization (24). However, this study found that the echo-enhanced submucosal blood flow signal was abundant and pathologically prompted inflammation, which is consistent with the results of Migaleddu et al. (23).
Intestinal stenosis manifests on ultrasound as thickening of the intestinal wall, a reduced intestinal lumen diameter (<1 cm), disappearance of intestinal peristalsis, and dilatation of the intestinal lumen (>2.5 cm) before the stenosis. When obstruction is present, the peristalsis of the stenosis is often hyperactive. In this study, ultrasound showed that the intestinal wall was uniform and hypoechoic, indicating intestinal inflammatory stenosis, while ultrasound showed that the uneven echo of the intestinal wall was more prone to fibrous intestinal stenosis. CEUS can accurately and reliably quantitatively evaluate intestinal inflammation, which is of great significance for the differentiation of inflammatory stenosis and fibrotic stenosis. Taking surgical pathological specimens as the reference standard, contrast enhancement of the bowel wall in patients with inflammatory stenosis was found to be significantly greater than that in patients with fibrotic stenosis (22). Elastography quantitative assessment of fibrotic stenosis is significantly correlated with fibrosis suggested by histopathology (25,26). The combination of CEUS and elastic ultrasound can provide clues for the clinical identification of stenosis components and further guide clinical decision-making. In this study, the stenosis score was used to identify the cause of intestinal stenosis in patients. The results showed that there were significant differences in shear wave velocity, Young’s modulus, PE, TTP, and AUC between patients with inflammatory stenosis and fibrotic stenosis. The presence of persistent inflammation in the fibrotic and narrow bowel wall may be the reason for the contradictory results of some studies. Wilkens et al. found no correlation between CEUS perfusion parameters, and inflammatory activity or histological grade of fibrosis, and believed that the thickness of the bowel wall had the best correlation with mobility and fibrosis of any parameter (27). CEUS is more accurate in the evaluation of the degree of acute inflammation. Elastic ultrasound can determine the presence of intestinal wall fibrosis by evaluating the stiffness of the intestinal wall. The combination of the two can better distinguish the degree of fibrosis and inflammation of intestinal stenosis in patients with CD (28). However, another study showed that the value of the CEUS parameter PE was moderately negatively correlated with that of fibrosis suggested by histopathology, and PE has no correlation with inflammation. There is no obvious correlation between elastic ultrasound and fibrosis suggested by histopathology. In addition, the study also showed that the main cause of wall thickening and narrowing is muscle hypertrophy, rather than the presence of fibrosis (29). This study used mucosal biopsy histopathological evaluation, which is less accurate than gross specimen pathological evaluation used in other studies (22). However, the preliminary results are consistent and need to be further verified by using more precise reference standards in future.
In order to improve the evaluation of inflammatory bowel disease by transabdominal ultrasound, the patient can be instructed to take the non-absorbable anechoic isotonic contrast agent polyethylene glycol to expand the intestinal cavity, which can improve the identification of the intestinal wall structure, especially the structure of the small intestine, and improve the accuracy of detection of intestinal lesion location, scope, mobility and complications.
Conclusions
Multi-modal ultrasound has advantages over single-modal ultrasound for the evaluation of the disease state of IBD. By combining multiple ultrasound technologies, more detailed clinical reference information can be obtained for comprehensive disease evaluation. In clinical practice, endoscopic evaluation, clinical symptom evaluation, CT, MR, and other auxiliary examination methods for IBD patients have certain advantages and disadvantages. Ultrasound, with its unique advantages and strong correlation with reference standards, is expected to become an important auxiliary examination method for IBD patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/apm-20-2162
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2162
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2162). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical committee of Tianjin Medical University General Hospital (No. IRB2020-WZ-163), and all research subjects signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:92-102. [PubMed]
- Atreya R, Neurath MF. Current and future targets for mucosal healing in inflammatory bowel disease. Visc Med 2017;33:82-8. [Crossref] [PubMed]
- Horsthuis K, Bipat S, Bennink R, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64-79. [Crossref] [PubMed]
- Ordas I, Rimola J, Rodriguez S, et al. Imaging of the colon in inflammatory bowel disease: ready for prime time? Curr Drug Targets 2012;13:1252-60. [Crossref] [PubMed]
- Gionchetti P, Dignass A, Danese S, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis 2017;11:135-49. [Crossref] [PubMed]
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 2017;11:649-70. [Crossref] [PubMed]
- Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol 1999;37:495-508. [PubMed]
- Drews BH, Barth TF, Hänle MM, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol 2009;19:1379-86. [Crossref] [PubMed]
- Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994;330:811-5. 2
- Schirin-Sokhan R, Winograd R, Tischendorf S, et al. Assessment of inflammatory and fibrotic stenoses in patients with Crohn’s disease using contrast-enhanced ultrasound and computerized algorithm: a pilot study. Digestion 2011;83:263-8. [Crossref] [PubMed]
- Parente F, Greco S, Molteni M, et al. Modern imaging of Crohn’s disease using bowel ultrasound. Inflamm Bowel Dis 2004;10:452-61. [Crossref] [PubMed]
- Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011;34:125-45. [Crossref] [PubMed]
- Calabrese E, Zorzi F, Onali S, et al. Accuracy of small-intestine contrast ultrasonography, compared with computed tomography enteroclysis, in characterizing lesions in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2013;11:950-5. [Crossref] [PubMed]
- Kucharzik T, Kannengiesser K, Petersen F. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol 2017;30:135-44. [PubMed]
- Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis 2008;40:860-6. [Crossref] [PubMed]
- Moreno N, Ripollés T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis 2014;8:1079-87. [Crossref] [PubMed]
- Sjekavica I, Barbarić-Babić V, Krznarić Z, et al. Assessment of Crohn’s disease activity by doppler ultrasound of superior mesenteric artery and mural arteries in thickened bowel wall: cross-sectional study. Croat Med J 2007;48:822-30. [Crossref] [PubMed]
- van Oostayen JA, Wasser MN, Griffioen G, et al. Diagnosis of Crohn’s ileitis and monitoring of disease activity: value of Doppler ultrasound of superior mesenteric artery flow. Am J Gastroenterol 1998;93:88-91. [Crossref] [PubMed]
- Giangregorio F, Bertone A, Fanigliulo L, et al. Predictive value of time-intensity curves obtained with contrast-enhanced ultrasonography (CEUS) in the follow-up of 30 patients with Crohn’s disease. J Ultrasound 2009;12:151-9. [Crossref] [PubMed]
- Romanini L, Passamonti M, Navarria M, et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol 2014;83:1317-23. [Crossref] [PubMed]
- Saevik F, Nylund K, Hausken T, et al. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:2029-37. [Crossref] [PubMed]
- Nylund K, Jirik R, Mezl M, et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med Biol 2013;39:1197-206. [Crossref] [PubMed]
- Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology 2009;137:43-52. [Crossref] [PubMed]
- Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn’s disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther 2003;18:749-56. [Crossref] [PubMed]
- Chen YJ, Mao R, Li XH, et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease. Inflamm Bowel Dis 2018;24:2183-90. [Crossref] [PubMed]
- Sconfienza LM, Cavallaro F, Colombi V, et al. In-vivo Axial-strain Sonoelastography Helps Distinguish Acutely-inflamed from Fibrotic Terminal Ileum Strictures in Patients with Crohn’s Disease: Preliminary Results. Ultrasound Med Biol 2016;42:855-63. [Crossref] [PubMed]
- Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of Contrast-enhanced Ultrasonography and Dynamic Contrast-enhanced MR Enterography in the Assessment of Transmural Activity and Fibrosis in Crohn’s Disease. J Crohns Colitis 2018;12:48-56. [Crossref] [PubMed]
- Thimm MA, Cuffari C, Garcia A, et al. Contrast-Enhanced Ultrasound and Shear Wave Elastography Evaluation of Crohn’s Disease Activity in Three Adolescent Patients. Pediatr Gastroenterol Hepatol Nutr 2019;22:282-90. [Crossref] [PubMed]
- Lu C, Gui X, Chen W, et al. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures. Inflamm Bowel Dis 2017;23:421-30. [Crossref] [PubMed]


