Painful boney metastases
Introduction
World health experts estimated that in 2008 there were over 12 million new cases of cancer diagnosed and 7.6 million deaths from cancer (1). It has been reported that up to 75-90% of patients with metastatic or advanced stage cancer will experience significant cancer-induced pain (2-5).
Approximately half or more of patients diagnosed with cancer may experience bone pain (6). Bone is the third most common site of metastatic disease. Breast, lung, and prostate cancers are collectively responsible for about 80 percent of secondary metastatic bone disease (7). Other common types of cancer, such as thyroid, lung, and renal cell carcinomas, also display significant osteotropism. Carinomas are more likely to metastasize to bone than sarcomas. The axial skeleton is seeded more than the appendicular skeleton, particularly due to the persistence of red bone marrow in the former. The ribs, pelvis and spine are generally involved early with distal bone involvement being infrequent. Batson's vertebral venous plexus permits malignant cells to enter the vertebral circulation without first passing through the lungs. Malignant cell survival with the development of spinal metastases may occur is common due to the sluggish blood flow in this plexus. In general, when a tumor grows in bone it may become more of a challenge to achieve a "cure" status, and it may cause devastating clinical complications, such as intractable severe pain, pathological fractures, spinal cord and nerve compression, hypercalcemia, and bone marrow aplasia, collectively referred as "skeletal-related events" (SRE) (7). Not all patients with bone metastases have pain, but about 83% of patients with osseous metastases complain of pain at some point with wide variation in pattern and severity (8,9).
CIBP often results in hospice or hospital admission and is associated with reduced quality of life, increased psychological distress and decreased physical and social functioning (10-12). With higher levels of disability, advanced illness and pain, comes an increased incidence of depression and anxiety (13).
CIBP does not exist as a single entity, but instead may be considered as a combination of background pain and breakthrough pain. Breakthrough pain (BTP) has been defined as 'a transitory exacerbation of pain experienced by the patient who has relatively stable and adequately controlled baseline pain' (14). Breakthrough pain can be divided into spontaneous pain at rest and incident pain (either volitional or non-volitional) (15,16). Breakthrough pain was present in 75% of cases of CIBP (17). Patients with breakthrough pain had greater interference on aspects of life (mood, relationships, sleep, activity, walking ability, work, enjoyment of life) than those with no breakthrough pain (P<0.01). Almost half of breakthrough pain episodes were rapid in onset (<5 min) and short in duration (<15 min). Forty-four per cent of patients with breakthrough pain had pain that was unpredictable (17). These clinical characteristics make the successful treatment of CIBP challenging. This has been supported by other studies that have shown that up to 45% of patients with CIBP report poor pain control (18,19).
Payne and Janjan recommend specialized interdisciplinary cancer center bone metastasis clinics for patients with painful osseous metastases, if available. Payne and Janjan have published an algorithm for assessment and management of these patients (20,21). Treatment strategies have employed various therapies for the treatment of painful osseous metastases including: Bisphosphonates (22), chemotherapeutic agents--mitoxantrone (a chemotherapeutic agent that inhibits DNA synthesis) (23), hormonal therapy, interventional, and surgical approaches (24). Additional agents may include systemic analgesics, steroids, radiation, (external beam radiation, radiopharmaceuticals), and ablation [radiofrequency ablation (RFA) and cryoablation], and intrathecal analgesics.
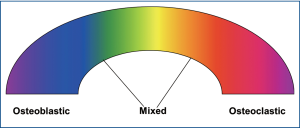
Metastatic bone disease is classified as osteolytic (e.g., breast) and osteblastic (e.g., prostate); however, usually lesions lie within a spectrum of these two entities (Figure 1). As denoted by their names, osteolytic metastases, which are considerably more common, are characterized by significant bone disruption due to augmented osteoclastic activity; on the contrary, osteoblastic metastases are characterized by overproduction of osseous tissue by activated osteoblasts (25).
Pathophysiology of bone metastases
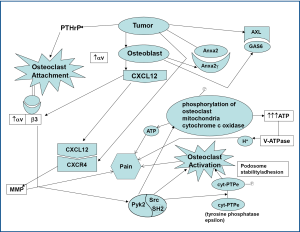
In order for bone metastases to develop, cancer cells first have to metastasize to the bone marrow which is mainly composed of hematopoietic stem cells (HSCs) residing in two different biological structures known as osteoblastic and vascular niches (26). Communications between osteoblasts as well as other tumor stromal cells and HSCs are mainly driven via chemoattractive factors such as the stromal-derived factor 1 (SDF-1) on stromal cells and its receptor CXCR4 on HSCs (27).
Communication between the tumor cells and bone marrow hematopoietic stem cells is vitally important in for the development of osseous metastases. A significant role in the interaction between cancer and bone is played by stromal-d factor 1 (SDF-1, also known as CXCL12) binding to CXCR4 with resultant CXCR4 signaling. The attachment/adherence of osteoclasts to bone/collagen is in large part due to αvβ3. This is facilitated by cathepsin K exposing the RGD sequence from collagen to αvβ3. Osteoclast activation appears to contribute to osteolytic lesions/erosions and pain. C-Src kinase activity is increased in response to integrin binding as well as RANKL/RANK interaction, and increased c-Src is involved in promoting osteoclast function/activation.
The development of bone metastases is a multi-step process which includes the following sequence of events: (I) tumor growth, detachment of cancer cells, and invasion of the tissue stroma; (II) neoangiogenesis; (III) escape from the tissue by intravasation; (IV) survival in the circulation; (V) chemoattraction and arrest (docking and locking) in the bone marrow endothelial vessel wall; (VI) extravasation; and (VII) establishment of the metastatic microenvironment (osteoblastic metastasis) via the cross-talk between the cancer and bone cells (28-31).
Pathophysiology of bone sesorption
Bone metastases may lead to pain via stimulation of nociceptors by algesic mediators [e.g., cytokines (Geis et al. demonstrated that evoked pain behavior and spinal glia activation is at least in part dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer) (32), prostaglandins, bradykinin, serotonin, substance P]. Involvement or invasion, stretching, or compression of pain-sensitive structures such as nerves, vasculature, and periosteum and microfractures of various joint structures may also lead to pain. Pain from osseous metastic lesions may also occur from mechanical instability of "weakened bone" or high intra-osseous pressures (>50 mmHg) (33).
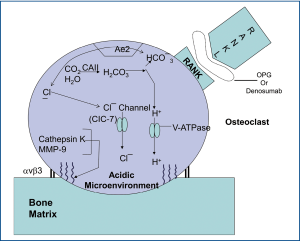
Although numerous contributing factors lead to the pain of osseous metastases, a significant portion of the pain seems to be related to osteoclastic bone resorption. Osteoclasts solubilize the mineral (e.g., hydroxyapatite) and degrade the organic matrix (e.g., type 1 collagen) with cysteine-proteinases. The bone resorption occurs in an acidic microenvironment produced by proton secretion via vacuolar H+-ATPases in osteoclastic membranes. The first step in the process of bone resorption is that the osteoclast adheres to the bone surface. This adherence is mediated by specific membrane receptors. Podosomes are osteoclastic processes that become the primary attachment sites to bone. The podosomes are made up of integrins and cytoskeletal proteins: Actin microfilaments surrounded by vinculin and talin (34).
The predominant attachment site is the vitronectin receptors (e.g., αvβ3 integrin), which recognizes the RGD (Arg-Gly-Asp) amino acide sequence in various bone matrix proteins (osteopontin, vitronectin, bone sialoprotein) (34). Integrin activation appears to result in Pyk2-dependent recruitment of c-Src to the plasma membrane and lead to c-Src activation and association with Pyk2 and subsequent c-Src-dependent phosphorylation of the nonreceptor isoform of tyrosine phosphatase epsilon (cyt-PTPe) at its C-terminal residue Y638, supports osteoclast adhesion and activation as well as proper structure, stability, and dynamics of podosomes (35).
A highly convoluted membrane area termed the ruffled border and sealing zone appears in the osteoclast during bone resorption. The accumulation of podosomes at the bone surface occurs first with ligand binding to the vitronectin receptor (34). Subsequently, a tight sealing zone is formed where osteoclastic acid and proteases reorganize elements to form a "double circle" of vinculin and talin around a core of F-actin (34).
In order to effectively "digest" inorganic bone matrix components (e.g., hydroxyapatite) at least two major factors are needed: (I) acid (e.g., HCI) and (II) energy (e.g., ATP). The osteoclats generate H+ and Cl– utilizing carbonic anhydrase II (CAII) that catalyzes conversion of carbon dioxide [CO2] and water [H2O] into carbonic acid [H2CO3], which in turn dissociates into hydrogen ion [H+] and bicarbonate [HCO3–] (36,37). The HCO3– ions are then exchanged for Cl– through the basolaterally located Anion Exchanger 2 (AE2) (38,39), providing the Cl- ions required for acidification [HCl] occurring in the resorption lacuna (Figure 2).
Inside the sealing zone, bone resorption is induced by active secretion of protons to the bone surface through a specialized vacuolar type ATPase (V-ATPase) requiring ATP, containing the a3 subunit (40-43) and passive transport of chloride through the chloride channel [ClC-7], also to the bone surface (Figure 2) (44-48). Hydrochloric acid lowers the pH to approximately 4.5, leading to dissolution of the inorganic matrix of bone (49).
Thus, involvement of vacuolar H+-ATPase and carbonic anhydrase are crucial to "digesting" bone with subsequent creation of osteolytic lesions. c-Src may contribute to bone resorption, in part by: (I) preventing the inhibitory effects of calcitonin on osteoclast function and facilitating osteoclast activation, (II) enhancing the normal organization of the osteoclast actin cytoskeleton and contributing to the formation of the "ruffled border" after c-Src is recruited to the plasma membrane, (III) facilitating podosome activities by promoting a shift from stable focal adhesions with actin stress fibers to more dynamic podosome assemblies, (IV) by phosphorylating cytochrome c oxidase within the mitochondria, thereby increasing cytochrome c oxidase activity, and subsequently contributing to the generation of high levels of ATP required for bone resorbing actions of osteoclasts (Figure 3) (50-52). The ATP produced by c-Src-induced cytochrome c oxidase activity may be utilized by V-ATPase to provide energy for the proton pump to secrete hydrogen ions by the bone surface. Furthermore, the ATP generated may also contribute to nociception via binding to purinergic receptors (P2X2/3 and P2X3).
Cleavage of the type I collagen fibers is mainly mediated by the cysteine proteinase cathepsin K, which is active at low pH (53-56), and performs almost complete removal of the type I collagen fibers (57). The MMPs are also involved in the degradation of the organic matrix of the bones; however, their precise role is remains uncertain (Figure 2). Targeting major processes involved in painful osseous metastases may lead to novel potential future therapeutic agents (Table 1).
Table 1
| Target | Process | Potential Therapy |
|---|---|---|
| CXCR4 | Communication (between tumor and hematopoetic stem cell) | CXCR4 Antagonists |
| αvβ3 | Attachment [between osteoclast (αvβ3) and bone/collagen (RGD)] | αvβ3 antagonists |
| Cathepsin K (exposes RGD) | Cathepsin K Inhibitors | |
| RANKL-RANK interaction | Osteoclast Activation | Denosumab |
| Prenylation of Src | Bisphosphonates | |
| Src | Src Inhibitors | |
| Src | Src→ATP ↔ binding to P2X3, P2X2/3 Energy ↓ Nociception |
Src Inhibitors |
| Vacuolar H+-ATPase | Bone Resorption-Acidic Microenvironment (proton secretion)-dissolution of Inorganic Matrix | Inhibitor of vacuolar H+-ATPase [V-ATPase] (e.g., bafilomycin A1) - subunit a3 |
| Carbonic Anhydrase | Carbonic Anhydrase Inhibitors | |
| CIC-7 (Chloride Channel) | Inhibitors of CIC-7 (Chloride Channel) | |
| Ae2 (Anion Exchanger) | Inhibitors of Ae2 (anion exchanger) | |
| Cathepsin K | Bone Resorption Proteolysis-removal of collagen fibers | Inhibitors of Cathepsin K |
| MMP-9 | Inhibitors of MMP-9 |
Nonpharmacologic approaches to the management of POM
Treatment strategies for painful osseous metastases include multiple nonpharmacologic approaches. These may include: physical medicine approaches, tai chi, yoga, stretching modalities, heat, cold, galvanic ultrasound, behavioral medicine approaches, cognitive behavioral therapy, mediation, hypnosis, relaxation techniques, guided imagery, and acupuncture. Bradt and colleagues performed a systematic review indicating that music interventions may have beneficial effects on anxiety, pain, mood, and QOL in people with cancer (58). Jane and colleagues conducted a randomized clinical trial was to compare the efficacy of massage therapy (MT) to a social attention control condition on pain intensity, mood status, muscle relaxation, and sleep quality in a sample (n=72) of Taiwanese cancer patients with bone metastases (59). MT was shown to have beneficial within- or between-subjects effects on pain, mood, muscle relaxation, and sleep quality.
Pharmacologic approaches to the management of POM
The "standard" or "traditional" pharmacologic approach to the treatment or palliation of painful osseous metastases follows the World Health Organization (WHO) analgesic stepladder guidelines approach to pain relief (60,61). An international WHO Expert Committee on cancer pain, chaired by Dr. Kathleen Foley of Memorial Sloan-Kettering Cancer Center, was convened in 1982, and in 1986 the WHO monograph Cancer Pain Relief was published (62). By 1993 it has been translated into 22 languages (62). The WHO guidelines have been prospectively and cross-culturally validated and shown to work well clinically (62). Zech et al. published the largest prospective trial of WHO guidelines to date and achieved favorable pain control in 76% of 2118 cancer patients who were treated over a 10-year interval (63). Analgesic agents which may play a role in the WHO guidelines approach include: Non-opioid analgesics [acetaminophen, traditional or nonselective nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitors], adjuvants [antidepressants, anticonvulsants, muscle relaxants, alpha-2 adrenergic agonists, n-methyl-d-aspartate (NMDA)receptor antagonists], and opioids/opioid-like analgesic agents.
Anti-inflammatory agents
NSAIDs appear to be particularly useful in patients with bone pain or pain related to inflammatory conditions and less useful in patients with neuropathic pain (64). However, although clinicians regard anti-inflammatory agents as important drugs for the treatment of CIBP, this has largely been based on experience rather than a strong evidence base; and the use of traditional [nonselective (NS)] NSAIDs in CIBP has been questioned due to the lack of robust, clinical evidence (65). Three randomized trials of NSAIDs in cancer pain did not separate out bone metastases, and six non-randomized trials mention bone metastases but did not record incident pain (66-73).
Eisenberg and colleagues performed a meta-analysis of the published randomized controlled trials to assess the efficacy and safety of nonsteroidal antiinflammatory drugs (NSAIDs) in the treatment of cancer pain by meta-analyses of the published randomized control trials (RCTs) (74). Twenty-five studies met inclusion criteria for analysis. Of these, 13 tested a single-dose effect, nine multiple-dose effects, and three both single- and multiple-dose effects of 16 different NSAIDs in a total of 1545 patients (74). Better pain relief was obtained from NSAIDs than placebo in three scores [summed pain intensity difference (SPID), peal pain relief (PPAR), and total pain relief (TOPAR)] based on five or six studies, and in another score peak pain intensity difference [PPID] based on eight studies. Single doses of placebo produced a 15% to 36% rate of analgesia, whereas NSAIDs resulted roughly twice as much analgesia (31% to 60%). All differences between NSAIDs and placebo comparisons were statistically significant (74). Pain was related to bone metastases in seven studies. Four studies enrolled patients with either malignant bone pain, non-bone malignant pain, or both, but results were not reported separately for bone-related and non-bone-related pain in any study. Three studies (75-77) examined the analgesic efficacy of NSAIDs specifically for malignant bone pain, but not for other types of malignant pain. Analgesic efficacy data were extractable from only two of these studies, but were not combinable because one was a single-dose trial (77) and the other a multiple-dose trial (75). The single-dose with ketoprofen study crossover reported a mean NSAID-induced PPID of 40% to 55% and SPID of 34% to 45%. The NSAID PPID in the multiple-dose study with naproxen 275 vs. 550 mg was 23% to 33% (74).
McNicol and colleagues performed a Cochrane review and evaluated forty-two trials involving 3,084 patients were included. Clinical heterogeneity of study methods and outcomes precluded meta-analyses and only supported a qualitative systematic review (78,79). They concluded that based upon limited data, NSAIDs appear to be more effective than placebo for cancer pain (7 out of 8 papers); clear evidence to support superior safety or efficacy of one NSAID over another is lacking; and trials of combinations of an NSAID with an opioid have disclosed either no difference (4 out of 14 papers), a statistically insignificant trend towards superiority (1 out of 14 papers), or at most a slight but statistically significant advantage (9 out of 14 papers), compared with either single entity. The short duration of studies undermines generalization of their findings on efficacy and safety of NSAIDs for cancer pain (78).
Cyclooxygenase (COX)-2 inhibitors may in theory be of greater therapeutic potential in well selected patients due to their anti-tumor/antiangiogenic properties (80,81); especially in patients at high risk of gastrointestinal complications and those at risk of bleeding. Studies have shown in the sarcoma model of bone cancer pain that chronic inhibition of COX-2 activity with selective COX-2 inhibitors resulted in significant attenuation of bone cancer pain behaviors [both spontaneous and movement-evoked pain] as well as many of the neurochemical changes suggestive of both peripheral and central sensitization (82). Microsomal prostaglandin E synthase-1 (mPGES-1) acts on COX-2 derived endoperoxide PGH2 to catalyze its isomerization PGE2. Thus, mPGES-1 inhibition may represent a therapeutic target to treating painful osseous metastases (83). Lumiracoxib (Cyclooxygenase-189; Prexige®), is a highly selective COX-2 inhibitor which is not approved in the United States, Canada, Australia, England, and in some other countries due to hepatic related adverse events. Compared with diclofenac, lumiracoxib has substantially reduced affinity for COX-1, being 300-fold less potent. The pKa of lumiracoxib is 4.3 and thus, lumiracoxib is predicted to be more effective in a low pH environment; which may potentially be beneficial for pain relief in sites of metastatic bone lesions, where the local environment is acidic in nature.
Steroids
Corticosteroids are commonly used for bone-related pain management which includes dexamethasone, methylprednisolone, and prednisone. Dexamethasone is often preferred orally because of its relatively high anti-inflammatory potency and low mineralcorticoid potency; therefore, dexamethasone has a lower risk of causing salt and water retention compared to equipotent doses of other corticosteroids. The mechanism of action of corticosteroid-induced analgesia is uncertain but may be related to decreasing tumor-related edema or inhibition of prostaglandin and leukotriene synthesis.
To date, only one study specifically addressed corticosteroid use for cancer-related bone pain. This study was a 14-day, randomized, double-blind, placebo-controlled, crossover study comparing 32 mg of oral methylprednisolone (MP) (16 mg twice daily) with placebo for symptoms in terminally ill cancer patients. After the initial 14 days, patients were continued on MP for a treatment period of 20 days. Pain intensity was significantly lower after MP compared with baseline or placebo in the crossover phase; 68% of patients responded that their pain control was better with MP compared to placebo by the end of the treatment phase. Furthermore, the findings of this study suggest that MP exerts its action rapidly, and the chances of obtaining better responses after 5 days of treatment are poor (84).
Antiepileptic drugs
Animal models of cancer pain have demonstrated that peripheral nerve destruction can take place in both skin (85) and bone (86). Additionally, sensitization of unmyelinated primary afferent fibers and damage to small and medium sized sensory neurons may occur. Metastatic tumor cells and/or tumor stromal cells in bone appear to lead to sensory nerve injury as evidenced by changes that include: Sprouting of sensory fibers into bone (87), increased expression of activating transcription factor-3 (ATF-3) in the nucleus of sensory neurons that innervate bone, as well as up-regulation of galanin and glial fibrillary acidic protein (GFAP) with hypertrophy of satellite cells surrounding ipsilateral dorsal root ganglion (DRG) sensory neuron cell bodies and ipsilateral DRG macrophage infiltration (88).
Gabapentin and pregabalin are voltage-gated calcium channel blockers believed to exert their effects at the alpha-2-delta-1 subunit. It has been demonstrated in animal studies, that gabapentin reverses dorsal horn changes associated with POM resulting in relief of spontaneous and movement-related pain (89). In a sarcoma model chronic treatment with gabapentin did not affect tumor growth, tumor-induced bone destruction or tumor-induced neurochemical reorganization in sensory neurons or spinal cord, but did attenuate both ongoing and movement-evoked bone cancer-related pain behaviors (86). These changes suggest that there is likely a neuropathic component which exists in conjunction with nociceptive and inflammatory components in painful osseous metastases. In animal models gabapentin demonstrated modulation of continuous and stimulus-related bone pain (89,90) it has been reported to be useful for the treatment of neuropathic cancer pain (91), and as a synergistic adjuvant to opioid analgesics. Caraceni and colleagues published an anecdotal report describing their treatment of six consecutive patients with incident pain caused by bone metastases with gabapentin not completely controlled by opioid medication. The addition of gabapentin was associated with significant clinical improvement of pain at rest and incident pain exacerbated by movement, which was sustained for up to 3 months (92). Clinical trials have been underway for assessing the effects of pregabalin on attenuating chronic bone pain related to metastases (93). As far as therapeutic agents in the class of "anticonvulsants", it is conceivable that topiramate may be an antiepileptic drug which is particularly well-suited for the treatment of painful osseous metastases, since in addition to its multiple mechanisms of action, it also possesses actions as a carbonic anhydrase inhibitor. Topiramate is a calcium channel blocker, sodium channel blocker, glutamic acid inhibitor; GABA facilitator and may affect the NMDA receptor complex. Adequate hydration is recommended due to the potential formulation of calcium phosphate renal stones.
Opioids
One of the major classes of agents for the pharmacologic management of POM is that of opioid analgesics. Preclinical research suggests that there may be varying efficacy for different opioids (94), however, clinically there does not appear to be any opioid that is better than any other opioid for the treatment of painful osseous metastases. Although some opioids may provide more analgesia than other opioids for a specific individual patient, currently, "trial and error" is the only way to determine this. Opioids are considered an effective therapy for background pain in CIBP. However, their usefulness in breakthrough pain is less clear. It appears to be vitally important to match the characteristics of the opioid utilized to treat BTP; to the type of BTP experienced. Immediate release oral morphine has, at best, an onset of action of about 30 min (95). This means that in patients with rapid-onset, short duration breakthrough pain, immediate release morphine will probably be ineffective. Furthermore, titration of opioids to doses that control episodes of breakthrough pain may result in unacceptable opioid side-effects (96). Newer, rapid-onset opioids have been developed with the aim of mirroring the temporal features of breakthrough pain.
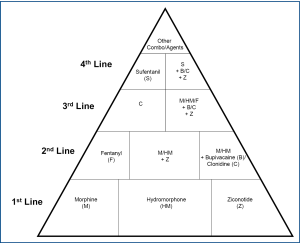
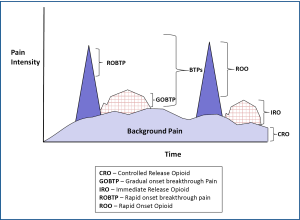
The author suggests a "triple opioid therapy (TOT) approach" to using opioid analgesics to treat painful osseous metastases. A triple opioid therapy approach utilizes three different opioid formulations [a controlled release opioid, an immediate release opioid, and a rapid onset opioid (ROO)]. Enteral or transdermal extended release (ER) or controlled release (CR) opioids are employed for "maintenance" therapy to control the baseline or background constant pain. The patient receiving TOT then evaluates breakthrough pain (BTP) episodes; (I) if a BTP episode seems relatively predictable and gradually intensifies over a half-hour or more [Gradual Onset Breakthrough Pain (GOBTP)] then it may be treated early with an immediate release (IR) opioid formulation, however, (II) if a BTP episode is unpredictable and/or the intensity suddenly increases rapidly [Rapid Onset Breakthrough Pain (ROBTP)], then it should be treated with a rapid-onset opioid (Figure 4).
Rapid-onset opioids FDA approved in the United States include: oral transmucosal fentanyl citrate (OTFC) [Atiq®], fentanyl buccal tablet (FBT) [Fentora®], fentanyl buccal soluble film (FBSF) [Onsolis®], sublingual fentanyl (SLF) [Abstral®], and fentanyl pectin nasal spray (FPNS) [Lazanda®] (97). Potential future rapid-onset opioids may include: Intranasal fentanyl spray (INFS) [Instanyl®] and fentanyl dry powder intrapulmonary inhaler [TAIFUN®] (97).
Bisphosphonates
Early-generation bisphosphonates (i.e., clodronate and etidronate) lack nitrogen and adhere to bone, where they are metabolized by osteoclasts. Metabolic products include cytotoxic ATP analogs that interfere with mitochondrial membrane potential and lead to osteoclast apoptosis (98,99). Later generation, nitrogen-containing bisphosphonates (i.e., pamidronate, ibandronate and zoledronate) inhibit osteoclasts by a different mechanism. They are internalized - but not metabolized - by osteoclasts, where they subsequently inhibit an enzyme called farnesyl pyrophosphate (FPP) synthase. FPP synthase is required for producing intermediates (e.g., isoprenoid lipids) necessary for post-translational modification (prenylation) of several small GTPases, including Ras, Rho and Rac. These small GTPases are required for proper cellular vesicle transport, without which osteoclasts cannot form the tight sealing zones or ruffled borders at the bone surface that are required for resorption (98,99). Additionally, nitrogen-contain bisphosphonates may lead to the accumulation of ispentyl pyrophosphate (IPP) which may be conjugated with adenosine monophosphate (AMP) to form an endogenous ATP analog triphosphoric acid 1-adenosine-5'-ylster 3-(3-methylbut 3-enyl) ester [ApppI] which may inhibit mitochondrial adenine nucleotide translocase (ANT) and cause osteoclast apoptosis (100). In the United States bisphosphonates not used for osteoporosis include Zoledronic acid (indicated for a range of solid tumors, with osseous metastases-- breast, prostate, non-small cell lung, renal, and others), Pamidronate (included for breast cancer and multiple myeloma), ibandronate (indicated for breast cancer), and Clodronate (not approved in U.S.).
Multiple studies have demonstrated the efficacy of bisphosphonates in reducing skeletal complications and pain from bone metastases (101-104). Intravenous zoledronic acid has demonstrated the broadest clinical activity (105). Zoledronate (zoledronic acid) is the most potent of the nitrogen containing bisphosphonates, displaying superior efficacy in inhibiting FPP synthase activity, reducing bone resorption and relieving pain when compared with other bisphosphonates, such as clodronate and pamidronate (99,106-109). Zoledronic acid is the only bisphosphonate that has statistically shown significant reductions in skeletal morbidity, including bone pain, in patients with metastatic prostate cancer (110). Fulfaro and colleagues demonstrated a relationship between a decrease in bone pain in 75% of patients and modification of C-telopeptide levels was identified in bone metastases from prostate cancer treated with zoledronic acid (111).
Zoledronate, in particular, has been reported to have direct antitumor properties in preclinical studies. It is capable of inducing tumor cell apoptosis (112), inhibiting cancer cell invasion (113) and limiting metastatic outgrowth in visceral tissues at extremely high doses (109). Zoledronate treatment has been associated with a decline in circulating levels of the potent pro-angiogenic molecule, VEGF, in cancer patients (114). Zoledronate -mediated reductions in VEGF levels were associated with increased time to a skeletal-related event, increased time to the progression of bone disease and longer time to the worsening of performance status (115). Zoledronic acid distributes and bonds to osseous tissues and has a triphasic post-infusion decline process with a terminal half-life of 146 hours. Prior to therapy initiation of zoledronate, a dental evaluation and subsequent follow-up are needed in efforts to monitor for the occurrence and risk of osteonecrosis of the jaw.
Zoledronic acid can cause flu-like symptoms that are manageable with standard treatment. Renal monitoring is recommended due to association with iatrogenic renal function deterioration. Use of zoledronic acid should be avoided in patients with a Clcr of <30 mL/min and caution should be utilized when using coledronate in patients with other nephrotoxic agents. Dose reductions should be followed according to the package information sheet for patients with renal dysfunction.
Hormonal approaches to the management of POM
Modulation of various hormonal pathways may be useful in the therapeutic strategies to combat POM in certain types of cancers.
Radiotherapy
External beam RT for osseous metastases may lead to improved analgesia, elimination or reduction of analgesic usage, functional improvement, such as increased ambulation, and reduction in the risk of fracture in weight-bearing bones.
Approximately 80% of patients may be successfully treated with sequential whole-skeleton radiation, in which 6-8 Gy is administered as a single fraction to either the upper and lower part of the body, followed by a second dose of 6-8 Gy, given 4-6 weeks later, to the remainder of the body (116). Most prospective randomized trials evaluating differences in the outcomes have shown that single fraction regimens (mostly 8 Gy) are at least equal in analgesic efficacy to the various fractionated regimens (117). These results have been confirmed in three meta- analyses (118-120). Wu et al. (118) included eight randomized trials (3,260 patients) in a meta-analysis, comparing 1×8 Gy single fraction radiotherapy with various multi-fraction regimens and found that all multi-fraction regimens were essentially equal to single fraction therapy. Dennis and colleagues found that patients suffering from painful bone metastases with an estimated survival of 3 months should still be considered for palliative radiotherapy (119).
Radiopharmaceuticals
Radiopharmaceuticals such as Strontium-89 chloride and Samarium-153 lexidronam provide several advantages over conventional external beam radiotherapy: (I) they can be administered intravenously, (II) they can treat multiple, diffuse sites with mild bone marrow depression, and (III) they cause fewer adverse side-effects such as nausea, vomiting, diarrhea, and tissue damage (24).
Figuls and colleagues updated a Cochrane Review to determine efficacy and safety of radioisotopes in patients with painful bone metastases. Their update includes 15 studies (1146 analyzed participants): Four (325 participants) already included and 11 new (821 participants). They found a small benefit of radioisotopes for complete relief [risk ratio (RR) 2.10, 95% CI 1.32 to 3.35; Number needed to treat to benefit (NNT)=5] and complete/partial relief (RR 1.72, 95% CI 1.13 to 2.63; NNT=4) in the short and medium term (eight studies, 499 participants) (120).
Interventional approaches to the management of POM
Ablative procedures
Patients with "relatively soft" osteolytic or mixed osteolytic/osteoblastic lesions may be amenable to focal ablative therapy [radiofrequency ablation (RFA) (121) or cryoablation] for focal painful metastic sites.
While cryoblation may effectively treat intact or sclerotic bone, RFA energy is poorly delivered into sclerotic or otherwise intact bone (122). An advantage of cryoablation, is that the zone of ablation is readily monitored with intermittent CT or MR imaging. Cryoablation also allows the simultaneous use of multiple cryoprobes, which allows complete ablation of large lesions (up to approximately 8 cm diameter) in a single session.
Vertebral augmentation procedures
Vertebral augmentation procedures such as percutaneous vertebroplasty and percutaneous kyphoplasty may provide relief in patients with pathologic vertebral body compression fractures that do not cause neurological deficits but severely compromise quality of life largely because of intractable pain, but also due to loss of independence, mobility, and function often with resulting isolation/loneliness (123).
Intrathecal therapies for POM
Intrathecal analgesia has emerged as a key therapeutic option for pain relief for patients who have failed other treatment avenues as well as patients with adequate analgesia on high dose enteral or parenteral therapy but with unacceptable side effects (124).
First-line intrathecal analgesics include morphine, sulfate, hydromorphone and ziconotide (125), however, there are other alternative agents as well (Figure 5) (124-126).
Potential future approaches to the management of POM
Inhibitors of the RANK-RANKL system
The RANK-RANKL system plays a fundamental role in the maturation and function of osteoclasts and thus in the development and progression of bone metastasis. Therefore, inhibition of this system has been evaluated as therapeutic target for the treatment of osteolytic diseases, including bone metastasis (25).
It appears that some of the pain from metastatic bone lesions may be secondary to the effects of osteoclastic activity, so that "shutting down" osteoclastic activity is paramount to incorporate in analgesic treatments. Osteoclast bone-resorbing activity is dependent on the binding of the (TNF family molecule osteoprotegerin ligand (OPGL) (127), which is expressed on activated T cells and osteoblasts, to a receptor termed receptor activator of nuclear factor κB (NF-κB), abbreviated RANK (127). RANK is expressed on osteoclast precursors and mature osteoclasts (128). Any treatment that impedes the OPGL-RANK interaction will impair RANK activation and therefore impair osteoclastic activity and bone resorption. Osteoprotegerin (OPG) is a soluble tumor necrosis factor receptor molecule that is secreted and binds to the RANK activating site of OPGL, acting as a "dummy" or "decoy" receptor and preventing OPGL from binding to and activating the osteoclast RANK receptor (Figure 2) (127,129,130).
Amgen created a recombinant Fc-OPG (AMGN-0007) to treat multiple myeloma and bone metastatic breast cancer. Results from the Phase I trial were encouraging, in that Fc-OPG was well tolerated and its inhibitory effects on bone resorption were similar to the bisphosphonate, pamidronate (131). However, due to the superior efficacy of their newer agent, denosumab (AMG-162) - a fully human monoclonal antibody that specifically neutralizes RANKL - thereby inhibiting bone resorption, and concerns regarding deleterious OPG-mediated protection from TRAIL mediated apoptosis in cancer cells, Amgen ceased further clinical development of AMGN-0007 (132).
Charles S. Cleeland, PhD, University of Texas M. D. Cancer Center, Houston, Texas, and colleagues elsewhere examined differences between patient-reported pain interference with daily functioning using data from a phase 3 trial that compared denosumab with zoledronic acid in women with advanced breast cancer and bone metastases; and their findings in December 2010 presented at the 33rd annual San Antonio Breast Cancer Symposium (SABCS) (133).
In the trial, patients completed the 11-point Brief Pain Inventory-Short Form (BPI-SF) to assess pain interference with general activity, walking, work, mood enjoyment of life, relations with others and sleep, and to assess pain severity. The analysis included 1,018 patients treated with denosumab and 1,011 patients treated with zoledronic acid. Results showed that time to improvement in pain interference with activity (PIWA) tended to occur more rapidly with denosumab than with zoledronic acid (a median of 70 vs. 86 days; P=0.09). Also, time to worsening PIWA tended to tended to be longer with denosumab than with zoledronic acid (median of 394 vs. 310 days; P=0.13). In women with no pain or only mild pain at enrollment, denosumab showed a trend for shorter time to improvement in PIWA and a longer time to worsening PIWA. Also, a shift in analgesic use from no or low analgesics to strong opioids occurred in fewer patients treated with denosumab.
Cannabinoid receptors (CB2)
Cannabinoid Receptor-2 (CB2) agonists have been shown to act as an analgesic in acute, chronic, and neuropathic pain and do not lead to effects seen with CB1 agonists (134-137). CB2 agonists not only produce antinociceptive and anti-inflammatory effects, but also have been shown to increase bone density (138,139). CB2 agonists increase the number of osteoblasts (bone forming cells) and inhibit the production of osteoclasts (bone destruction cells) resulting in an overall increase in bone integrity (138). CB2 knockout mice experience accelerated trabecular bone loss and cortical expansion further demonstrating the importance of the endogenous CB2 system in the mediation of skeletal maintenance (138).
The CB2 agonist, AM1241, attenuates spontaneous pain behaviors in a murine bone cancer model (140). Acute treatment with the CB2 agonist, AM1241, attenuated bone cancer induced spontaneous and evoked pain; which was blocked by the CB2 antagonist SR144528. The CB2 agonist, AM1241, attenuated evoked pain behaviors in a murine bone cancer model. Tactile allodynia and movement-evoked pain were tested. AM1241 (i.p) treatment blocked tactile allodynia in cancer-induced mice compared to cancer-induced mice treated with vehicle on days 10 and 14. AM1241 (i.p) treatment significantly alleviate movement-evoked pain on day 14 in tumor-bearing mice treated with AM1241 when compared to cancer induced mice treated with vehicle (140).
S-NACH
Heparin is an unbranched, acidic glycosaminoglycan rich in N- and 0-sulfate groups that is synthesized by mast cells as a component of high molecular weight proteoglycans. Heparin molecules are composed of alternating residues of D-glucosamine (or N-acetyl-glucosamine) and uronic acid (L-iduronic or D-glucuronic acid) (141). Heparin acts as an anticoagulant by accelerating the inhibition of thrombin and other coagulation enzymes by the circulating protease inhibitor, antithrombin (also called antithrombin III or AT-III) (142). However, a modified heparin S-NACH (sulfated non-anticoagulant heparin) which is devoid of AT binding and inhibition of AT-dependent coagulation factor (e.g., factor Xa and IIa) can be utilized in high doses without fear of bleeding or hemorrhagic adverse effects.
Mounting evidence indicates that P-selectin plays a crucial role during hematogenous metastasis, and P-selectin has been shown to bind to several human cancers and human cancer-derived cell lines, such as colon cancer, lung cancer, breast cancer, malignant melanoma, gastric cancer (143,144). P-selectin plays roles mainly in the initial process of tumor cell adhesion to platelets (145,146). Therefore, it is conceivable that blocking P-selectin on platelets with antagonists can effectively inhibit the formation of tumor cell-platelet emboli complexes and successfully prevent hematogenous metastasis. Several studies have demonstrated that heparin and heparan sulfate were ligands for P-selectin and could block its binding to carbohydrate ligands (147). Heparin and heparin-like oligosaccharides can inhibit P-selectin binding to SLex-related compounds (148). Inhibition of P-selectin binding results in inhibition of the inflammatory response and may attenuate tumor metastasis (147,149). Li and colleagues have suggested that the critical antimetastatic effects by heparin are mediated primarily via interference with P-selectin mediated binding (150,151).
All three selectins can bind sialylated, fucosylated, or in some cases, sulfated glycans on glycoproteins, glycolipids, and proteoglycans. The tetrasaccharides sLex and sLea have been identified as the minimal ligands for all three types of selectins.
Each of the selectins can bind to slalyl-Lewis x (sLex), a sialylated and fucosylated tetrasaccharide found as a terminal structure of sugar chains on glycoproteins and glycolipids expressed by a variety of cell types, including neutrophils, monocytes, and colon cancer cells (152-156).
Intravenously administered heparin tetrasaccharides diminished influx of neutrophils into the peritoneal cavities of thioglycollate-treated mice, and demonstrated anti-inflammatory activity in vivo (149).
Vik and colleagues showed that both unfractionated heparin (UFH) and high-dose LMWH (dalteparin) increase plasma osteoprotegerin (OPG) levels likely by mobilizing and attracting OPG into the vascular compartment (157).
Ariyoshi and colleagues investigated the effects of Glycosaminoglycans (GAGs) on mouse monocytic cell line in regard to their differentiation, proliferation, and function in vitro. RAW 264.7 cells were cultured with receptor activator of NF-kappaB ligand (RANKL) and various GAGs (158). Heparin suppressed TRAP-positive multinucleated cell formation and TRAP activity induced by RANKL, whereas the other GAGs showed no effects on osteoclast differentiation. Heparin also inhibited the formation of resorption pits, while the others did not. Heparin reduced the level of c-Src protein in RAW 264.7 cells stimulated with RANKL. Heparin and RANKL were subjected by HiTrap heparin column chromatography and each fraction was collected. Western blotting analysis revealed the expression of RANKL in the fraction bound to heparin. The binding of RANKL and heparin was confirmed by quartz-crystal microbalance. These results indicate that the inhibitory effect of heparin toward osteoclastogenesis induced by RANKL is due to the binding of heparin to RANKL (158). This RANKL-Heparin binding appears to interfere with osteoclast formation and function.
Data revealed that heparin inhibited osteoclastogenesis in three animal models, which was confirmed by a decrease in mRNA expression of osteoclastic markers and by an inhibition of the bone resorption capacity. Baud'huin and colleagues also demonstrated in RAW 264.7 cells that other families of GAGs different from heparin inhibited RANKL-induced osteoclastogenesis, and that this inhibition was dependent on the length and the level of sulfation of GAGs. They found that heparin did not bind to RANKL and did not modulate RANKL signaling. Heparin acted at 2 distinct steps of osteoclastogenesis from human CD14(+) cells: First, heparin strongly decreased the adherence of osteoclast precursors, and secondly inhibited osteoclasts to spread and to be active. Furthermore, the second action of heparin was reversible as the removal of heparin at the end of the culture time allowed the condensed cells to spread out and showed the formation of morphological active osteoclasts (159).
Yee and colleagues treated C57BL/6 mice were treated with increasing doses of unfractionated heparin (15, 20, or 25 units/mouse) 30 min prior to the left ventricular injection of GFP-transfected B16F10 melanoma cells (160). Heparin's effect on tumor burden and bone strength was then quantified 14 days later by bone histomorphometry and biomechanical testing, respectively. Based on histomorphometric analysis of the femurs, injection of GFP transfected melanoma cells resulted in a 37% decrease in cancellous bone volume and a 68% increase in osteoclast surface. This was associated with a 13% reduction in bone strength as measured by biomechanical testing. However, when the mice were first pre-treated with 25 units of heparin, tumor burden was decreased by 73% and tumor cell-dependent decreases in both cancellous bone volume and bone strength were prevented (160). Yee et al. concluded that heparin inhibitors tumor cells to metastasize to bone and that as such, prevents tumor cell-induced decreases in bone strength (160).
Heparin may also attenuate the targeting of tumor cells to bone. Bone is highly vascularized, and heparin may prevent tumor cell binding to the surface of sinusoidal endothelium. Thus, many tumor cells express sialyl Lewisx on their surface. Sialyl Lewisx is the ligand for P-selectin and is expressed on the surface of activated endothelial cells. Heparin blocks P-selectin interactions with its ligand and this may prevent tumor cell-endothelial cell interactions (151,161-164). Without P-selectin binding, tumor cell adhesion to endothelium via other molecules such as integrins is either delayed or does not occur. S-NACH may combat painful osseous metastases via multiple mechanisms (Table 2).
Table 2
| Interference with P-selectin mediated binding with resultant ↓adherence/↓metastases/↓inflammation |
| ↓Neutrophil accumulation |
| ↑Circulating osteoprotegerin (OPG) |
| Heparin – RANKL binding with subsequent interference with osteoclast formation and function |
| ↓c-Src level and/or activity (likely indirect effect) |
| ↓Mast cell degranulation/↓histamine availability secondary to histamine binding to heparin (S-NACH) |
| Interference with IL-1β-induced: (I) phosphorylation of p38MAPK/ERK (II) nuclear translocation of NF-κB (III) upregulation of MMP-3 synthesis |
Potential future therapeutic agents
Additionally, it is conceivable that potential future therapeutic agents may include nerve growth factor (NGF) inhibitors, inhibitors of cathepsin K, Src inhibitors, inhibitors of MMP-9 αvβ3 antagonists, CXCR4 antagonists, endothelin-A receptor antagonists (e.g., atrasentan) (165), and tyrosine kinase inhibitors [e.g., cabozantinib (XL184)].
Conclusions
Metastatic disease to the bone has been a crippling devastating complication of various cancers, leaving patients bedridden or wheelchair-bound and victims of suffering with unbearable pain. Knowledge surrounding the pathophysiology of painful osseous metastases is rapidly changing. Treatment approaches continue to be introduced into practice as they are approved. The advent of intravenous bisphosphonates has not only given clinicians another agent to reduce pain but also to reduce and/or postpone the risk of "skeletal-related events". RANK-L inhibition with denosumab represents a new therapeutic approach to also prevent or delay "skeletal-related events" as well as reduce pain. A greater understanding of the pathophysiology of painful osseous metastases may lead to improved analgesia with minimal adverse effects by utilizing tailor-made selective targeted therapy. It is hoped that potential future therapeutic agents for the treatment of painful osseous metastases may revolutionize current pharmacologic approaches and lead to improved patient outcomes with better quality of life.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
Footnote
No potential conflict of interest.
References
- Boyle P, Levin B. World cancer report 2008. International Agency for Research on Cancer. Lyon: France, 2008.
- Berruti A, Dogliotti L, Bitossi R, et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J Urol 2000;164:1248-53.
- Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247-57.
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49.
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain 2007;132:312-20.
- Farhanghi M, Holmes RA, Volkert WA, et al. Samarium-153-EDTMP: pharmacokinetic, toxicity and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J Nucl Med 1992;33:1451-8.
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61-6.
- Delaney A, Fleetwood-Walker SM, Colvin LA, et al. Translational medicine: cancer pain mechanisms and management. Br J Anaesth 2008;101:87-94.
- Walley J, Colvin LA, Fallon MT, et al. Characterisation of cancer-induced bone pain. Proc The British Pain Society 2006:A45.
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94.
- Neville-Webbe H, Coleman RE. The use of zoledronic acid in the management of metastatic bone disease and hypercalcaemia. Palliat Med 2003;17:539-53.
- Rustøen T, Moum T, Padilla G, et al. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage 2005;30:234-42.
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997;69:1-18.
- Portenoy RK, Forbes K, Lussier D, et al. Difficult pain problems: an integrated approach. In: Doyle D, et al, (eds). Oxford textbook of palliative medicine. Oxford, Oxford University Press;2004:438-58.
- Colvin L, Fallon M. Challenges in cancer pain management--bone pain. Eur J Cancer 2008;44:1083-90.
- Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev 1998;24:425-32.
- Laird BJ, Walley J, Murray G, et al. What is the key question in the assessment of cancer induced bone pain: results from a characterization study. London: British Pain Society Annual Scientific Meeting,2009.
- de Wit R, van Dam F, Loonstra S, et al. The Amsterdam Pain Management Index compared to eight frequently used outcome measures to evaluate the adequacy of pain treatment in cancer patients with chronic pain. Pain 2001;91:339-49.
- Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247-57.
- Payne R, Janjan N. Management of metastatic bone pain. Assessment and treatment of cancer pain. In: Payne R, Patt RB, Hill CS (eds). Progress in Pain Research and Management. Vol 12. Seattle:IASP Press;1998:269-73.
- Janjan NA, Payne R, Gillis T, et al. Presenting symptoms in patients referred to a multidisciplinary clinic for bone metastases. J Pain Symptom Manage 1998;16:171-8.
- Fulfaro F, Casuccio A, Ticozzi C, et al. The role of bisphosphonates in the treatment of painful metastatic bone disease: a review of phase III trials. Pain 1998;78:157-69.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14:1756-64.
- Smith H, Navani A, Fishman SM. Radiopharmaceuticals for palliation of painful osseous metastases. Am J Hosp Palliat Care 2004;21:303-13.
- Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: Molecular mechanisms and novel therapeutic interventions. Med Res Rev 2010; [Epub ahead of print].
- Santini D, Galluzzo S, Zoccoli A, et al. New molecular targets in bone metastases. Cancer Treat Rev 2010;36:S6-S10.
- Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 2006;38:497-508.
- Koutsilieris M. Osteoblastic metastasis in advanced prostate cancer. Anticancer Res 1993;13:443-9.
- Koutsilieris M. Skeletal metastases in advanced prostate cancer: cell biology and therapy. Crit Rev Oncol Hematol 1995;18:51-64.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7.
- Msaouel P, Pissimissis N, Halapas A, et al. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab 2008;22:341-55.
- Geis C, Graulich M, Wissmann A, et al. Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience 2010;169:463-74.
- Smith, HS. Novel Analgesic Approaches to Painful Bone Metastases. In: Smith, HS, (ed). Drugs for Pain. Philadelphia: Hanley and Belfus; 2003:489-497.
- Galasko CS. Mechanisms of lytic and blastic metastatic disease of bone. Clin Orthop Relat Res 1982;20-7.
- Granot-Attas S, Luxenburg C, Finkelshtein E, et al. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol Biol Cell 2009;20:4324-34.
- Sly WS, Hewett-Emmett D, Whyte MP, et al. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 1983;80:2752-6.
- Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med 2004;351:2839-49.
- Jansen I, De Vries T, Ravesloot J, et al. Loss of anion exchanger 2 (Ae2) in mice results in osteopetrosis. J Bone Miner Res ;21:S68.
- Teti A, Blair HC, Teitelbaum SL, et al. Cytoplasmic pH regulation and chloride/bicarbonate exchange in avian osteoclasts. J Clin Invest 1989;83:227-33.
- Frattini A, Orchard PJ, Sobacchi C, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 2000;25:343-6.
- Kornak U, Schulz A, Friedrich W, et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 2000;9:2059-63.
- Li YP, Chen W, Liang Y, et al. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet 1999;23:447-51.
- Scimeca JC, Franchi A, Trojani C, et al. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 2000;26:207-13.
- Blair HC, Teitelbaum SL, Ghiselli R, et al. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989;245:855-7.
- Blair HC, Teitelbaum SL, Tan HL, et al. Passive chloride permeability charge coupled to H(+)-ATPase of avian osteoclast ruffled membrane. Am J Physiol 1991;260:C1315-24.
- Henriksen K, Gram J, Schaller S, et al. Characterization of osteoclasts from patients harboring a G215R mutation in ClC-7 causing autosomal dominant osteopetrosis type II. Am J Pathol 2004;164:1537-45.
- Karsdal MA, Henriksen K, Sørensen MG, et al. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol 2005;166:467-76.
- Kornak U, Kasper D, Bösl MR, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001;104:205-15.
- Baron R, Neff L, Louvard D, et al. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol 1985;101:2210-22.
- Miyazaki T, Neff L, Tanaka S, et al. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol 2003;160:709-18.
- Miyazaki T, Sanjay A, Neff L, et al. Src kinase activity is essential for osteoclast function. J Biol Chem 2004;279:17660-6.
- Miyazaki T, Tanaka S, Sanjay A, et al. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol 2006;16:68-74.
- Bossard MJ, Tomaszek TA, Thompson SK, et al. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J Biol Chem 1996;271:12517-24.
- Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 1999;14:1654-63.
- Nishi Y, Atley L, Eyre DE, et al. Determination of bone markers in pycnodysostosis: effects of cathepsin K deficiency on bone matrix degradation. J Bone Miner Res 1999;14:1902-8.
- Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A 1998;95:13453-8.
- Everts V, Delaissé JM, Korper W, et al. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res 2002;17:77-90.
- Bradt J, Dileo C, Grocke D, et al. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 2011;CD006911.
- Jane SW, Chen SL, Wilkie DJ, et al. Effects of massage on pain, mood status, relaxation, and sleep in Taiwanese patients with metastatic bone pain: a randomized clinical trial. Pain 2011;152:2432-42.
- Bruera E, Kim HN. Cancer pain. JAMA 2003;290:2476-9.
- Hanks GW. The pharmacological treatment of bone pain. Cancer Surv 1988;7:87-101.
- Burton AW, Cleeland CS. Cancer pain: progress since the WHO guidelines. Pain Pract 2001;1:236-42.
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65-76.
- Vielhaber A, Portenoy RK. Advances in cancer pain management. Hematol Oncol Clin North Am 2002;16:527-41.
- Urch C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med 2004;18:267-74.
- Estapé J, Viñolas N, González B, et al. Ketorolac, a new non-opioid analgesic: a double-blind trial versus pentazocine in cancer pain. J Int Med Res 1990;18:298-304.
- Fuccella LM, Conti F, Corvi G, et al. Double-blind study of the analgesic effect of indoprofen (K 4277). Clin Pharmacol Ther 1975;17:277-83.
- Minotti V, De Angelis V, Righetti E, et al. Double-blind evaluation of short-term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain 1998;74:133-7.
- Minotti V, Patoia L, Roila F, et al. Double-blind evaluation of analgesic efficacy of orally administered diclofenac, nefopam, and acetylsalicylic acid (ASA) plus codeine in chronic cancer pain. Pain 1989;36:177-83.
- Stambaugh J, Drew J. A double-blind parallel evaluation of the efficacy and safety of a single dose of ketoprofen in cancer pain. J Clin Pharmacol 1988;28:S34-9.
- Staquet MJ. A double-blind study with placebo control of intramuscular ketorolac tromethamine in the treatment of cancer pain. J Clin Pharmacol 1989;29:1031-6.
- Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. J Clin Pharmacol 1988;28:S47-54.
- Ventafridda V, Martino G, Mandelli V, et al. Indoprofen, a new analgesic and anti-inflammatory drug in cancer pain. Clin Pharmacol Ther 1975;17:284-9.
- Eisenberg E, Berkey CS, Carr DB, et al. Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: a meta-analysis. J Clin Oncol 1994;12:2756-65.
- Levick S, Jacobs C, Loukas DF, et al. Naproxen sodium in treatment of bone pain due to metastatic cancer. Pain 1988;35:253-8.
- Stambaugh JE Jr, Drew J. The combination of ibuprofen and oxycodone/acetaminophen in the management of chronic cancer pain. Clin Pharmacol Ther 1988;44:665-9.
- Sacchetti G, Camera P, Rossi AP, et al. Injectable ketoprofen vs. acetylsalicylic acid for the relief of severe cancer pain: a double-blind, crossover trial. Drug Intell Clin Pharm 1984;18:403-6.
- McNicol E, Strassels SA, Goudas L, et al. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev 2005;CD005180.
- McNicol E, Strassels S, Goudas L, et al. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol 2004;22:1975-92.
- Sheng H, Shao J, Kirkland SC, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997;99:2254-9.
- Sumitani K, Kamijo R, Toyoshima T, et al. Specific inhibition of cyclooxygenase-2 results in inhibition of proliferation of oral cancer cell lines via suppression of prostaglandin E2 production. J Oral Pathol Med 2001;30:41-7.
- Sabino MA, Ghilardi JR, Jongen JL, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res 2002;62:7343-9.
- Isono M, Suzuki T, Hosono K, et al. Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci 2011;88:693-700.
- Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751-4.
- Cain DM, Wacnik PW, Turner M, et al. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci 2001;21:9367-76.
- Peters CM, Ghilardi JR, Keyser CP, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol 2005;193:85-100.
- Halvorson KG, Sevcik MA, Ghilardi JR, et al. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain 2006;22:587-600.
- Jimeenez-Andrade JMJ, Mantyh P. Cancer Pain. In: Kruger L, Light AR (eds). Transitional Pain Research: From Mouse to Man. Boca Raton, FL:CRC Press;2010:1-22.
- Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology 2005;102:132-40.
- Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med 2000;1:303-9.
- Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol 2004;22:2909-17.
- Caraceni A, Zecca E, Martini C, et al. Gabapentin for breakthrough pain due to bone metastases. Palliat Med 2008;22:392-3.
- Available online: http://clinicaltrials.gov. Accessed April 2009. NCT00381095.
- Kato A, Minami K, Ito H, et al. Oxycodone-induced analgesic effects in a bone cancer pain model in mice. Oncology 2008;74:55-60.
- Bailey F, Farley A. Oral opioid drugs. In: Davies A (ed). Cancer-related breakthrough pain. Oxford: Oxford University Press; 2006:43-55.
- Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 1999;81:129-34.
- Smith HS. Rapiod onset opioids in palliative medicine. Ann Palliat Med 2012;1:45-52.
- Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol 2009;6:163-74.
- Green JR. Bisphosphonates: preclinical review. Oncologist 2004;9:3-13.
- Mönkkönen H, Auriola S, Lehenkari P, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol 2006;147:437-45.
- Finley RS. Bisphosphonates in the treatment of bone metastases. Semin Oncol 2002;29:132-8.
- Purohit OP, Anthony C, Radstone CR, et al. High-dose intravenous pamidronate for metastatic bone pain. Br J Cancer 1994;70:554-8.
- Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol 2002;20:3719-36.
- Pistevou-Gombaki K, Eleftheriadis N, Sofroniadis I, et al. Palliative treatment of painful bone metastases from non-Hodgkin lymphoma with disodium pamidronate. J Exp Clin Cancer Res 2002;21:429-32.
- Santini D, Fratto ME, Vincenzi B, et al. Zoledronic acid in the management of metastatic bone disease. Expert Opin Biol Ther 2006;6:1333-48.
- Clemons MJ, Dranitsaris G, Ooi WS, et al. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol 2006;24:4895-900.
- Amir E, Whyne C, Freedman OC, et al. Radiological changes following second-line zoledronic acid treatment in breast cancer patients with bone metastases. Clin Exp Metastasis 2009;26:479-84.
- Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 2000;60:2949-54.
- Hiraga T, Williams PJ, Ueda A, et al. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res 2004;10:4559-67.
- Furlow B. Zoledronic acid palliation in bone-metastatic breast cancer. Lancet Oncol 2006;7:894.
- Fulfaro F, Leto G, Badalamenti G, et al. The use of zoledronic acid in patients with bone metastases from prostate carcinoma: effect on analgesic response and bone metabolism biomarkers. J Chemother 2005;17:555-9.
- Rachner TD, Singh SK, Schoppet M, et al. Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Lett 2010;287:109-16.
- Woodward JK, Neville-Webbe HL, Coleman RE, et al. Combined effects of zoledronic acid and doxorubicin on breast cancer cell invasion in vitro. Anticancer Drugs 2005;16:845-54.
- Santini D, Vincenzi B, Galluzzo S, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res 2007;13:4482-6.
- Vincenzi B, Santini D, Dicuonzo G, et al. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res 2005;25:144-51.
- Poulter CA, Cosmatos D, Rubin P, et al. A report of RTOG 8206: a phase III study of whether the addition of single dose hemibody irradiation to standard fractionated local field irradiation is more effective than local field irradiation alone in the treatment of symptomatic osseous metastases. Int J Radiat Oncol Biol Phys 1992;23:207-14.
- Jeremic B. Single fraction external beam radiation therapy in the treatment of localized metastatic bone pain. A review. J Pain Symptom Manage 2001;22:1048-58.
- Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594-605.
- Dennis K, Wong K, Zhang L, et al. Palliative radiotherapy for bone metastases in the last 3 months of life: worthwhile or futile? Clin Oncol (R Coll Radiol) 2011;23:709-15.
- Roqué I, Figuls M, Martinez-Zapata MJ, Scott-Brown M, et al. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev 2011;CD003347.
- Nazario J, Hernandez J, Tam AL. Thermal ablation of painful bone metastases. Tech Vasc Interv Radiol 2011;14:150-9.
- Dupuy DE, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol 2000;175:1263-6.
- Tancioni F, Lorenzetti MA, Navarria P, et al. Percutaneous vertebral augmentation in metastatic disease: state of the art. J Support Oncol 2011;9:4-10.
- Smith HS, Deer TR, Staats PS, et al. Intrathecal drug delivery. Pain Physician 2008;11:S89-S104.
- Deer T, Krames ES, Hassenbusch SJ, et al. Polyanalgesic consensus conference 2007: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation 2007;10:300-28.
- Newsome S, Frawley BK, Argoff CE. Intrathecal analgesia for refractory cancer pain. Curr Pain Headache Rep 2008;12:249-56.
- Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999;402:304-9.
- Hsu H, Lacey DL, Dunstan CR, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A 1999;96:3540-5.
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309-19.
- Thompson SW, Tonge D. Bone cancer gain without the pain. Nat Med 2000;6:504-5.
- Body JJ, Greipp P, Coleman RE, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 2003;97:887-92.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007;9:S7.
- Cleeland CS, Patrick DL, Fallowfield L, et al. Comparing the Effects of Denosumab and Zoledronic Acid on Pain Interference With Daily Functioning in a Randomized Phase 3 Trial of Patients With Breast Cancer and Bone Metastases. Presented at 33rd Annual San Antonio Breast Cancer Symposium (SABCS). Abstract P1-13-01. San Antonio, Texas; December 12, 2010.
- Malan TP Jr, Ibrahim MM, Lai J, et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol 2003;3:62-7.
- Ibrahim MM, Porreca F, Lai J, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A 2005;102:3093-8.
- Ibrahim MM, Rude ML, Stagg NJ, et al. CB2 cannabinoid receptor mediation of antinociception. Pain 2006;122:36-42.
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem 2007;14:917-36.
- Ofek O, Karsak M, Leclerc N, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A 2006;103:696-701.
- Karsak M, Cohen-Solal M, Freudenberg J, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 2005;14:3389-96.
- Lozano-Ondoua AN, Wright C, Vardanyan A, et al. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci 2010;86:646-53.
- Lane D, Lindahl V (eds). Heparin. Chemical and Biological Properties, Clinical Applications. Boca Raton, FL:CRC;1989.
- Bourin MC, Lindahl U. Glycosaminoglycans and the regulation of blood coagulation. Biochem J 1993;289:313-30.
- Hariri G, Zhang Y, Fu A, et al. Radiation-guided P-selectin antibody targeted to lung cancer. Ann Biomed Eng 2008;36:821-30.
- Monzavi-Karbassi B, Stanley JS, Hennings L, et al. Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int J Cancer 2007;120:1179-91.
- Napier SL, Healy ZR, Schnaar RL, et al. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J Biol Chem 2007;282:3433-41.
- Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer. Metast Rev 2008;27:19-30.
- Varki NM, Varki A. Heparin inhibition of selectin-mediated interactions during the hematogenous phase of carcinoma metastasis: rationale for clinical studies in humans. Semin Thromb Hemost 2008;28:53-66.
- Koenig A, Norgard-Sumnicht K, Linhardt R, et al. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest 1998;101:877-89.
- Nelson RM, Cecconi O, Roberts WG, et al. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 1993;82:3253-8.
- Lever R, Hoult JR, Page CP. The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium in vitro. Br J Pharmacol 2000;129:533-40.
- Ludwig RJ, Alban S, Bistrian R, et al. The ability of different forms of heparins to suppress P-selectin function in vitro correlates to their inhibitory capacity on bloodborne metastasis in vivo. Thromb Haemost 2006;95:535-40.
- Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 1992;258:964-9.
- Bevilacqua MP, Nelson RM. Selectins. J Clin Invest 1993;91:379-87.
- Fukuda M, Spooncer E, Oates JE, et al. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J Biol Chem 1984;259:10925-35.
- Macher BA, Klock JC. Isolation and chemical characterization of neutral glycosphingolipids of human neutrophils. J Biol Chem 1980;255:2092-6.
- Symington FW, Hedges DL, Hakomori S. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible HL-60 myeloid leukemia line. J Immunol 1985;134:2498-506.
- Vik A, Brodin E, Sveinbjørnsson B, et al. Heparin induces mobilization of osteoprotegerin into the circulation. Thromb Haemost 2007;98:148-54.
- Ariyoshi W, Takahashi T, Kanno T, et al. Heparin inhibits osteoclastic differentiation and function. J Cell Biochem 2008;103:1707-17.
- Baud'huin M, Ruiz-Velasco C, Jego G, et al. Glycosaminoglycans inhibit the adherence and the spreading of osteoclasts and their precursors: role in osteoclastogenesis and bone resorption. Eur J Cell Biol 2011;90:49-57.
- Yee CK, Butcher M, Zeadin M, et al. Inhibition of osteolytic bone metastasis by unfractionated heparin. Clin Exp Metastasis 2008;25:903-11.
- Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparins--correlation with selectin inhibition, not antithrombotic activity. Clin Cancer Res 2005;11:7003-11.
- Borsig L. Selectins facilitate carcinoma metastasis and heparin can prevent them. News Physiol Sci 2004;19:16-21.
- Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J 1997;14:577-84.
- Borsig L, Wong R, Feramisco J, et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A 2001;98:3352-7.
- Cella D, Petrylak DP, Fishman M, et al. Role of quality of life in men with metastatic hormone-refractory prostate cancer: how does atrasentan influence quality of life? Eur Urol 2006;49:781-9.