Risk factors of hypermagnesemia in end-stage cancer patients hospitalized in a palliative care unit
Introduction
Normally, an adult individual possesses about 25 g of magnesium, which is necessary for life-sustaining functions such as enzymatic action, transporters, and the synthesis of nucleic acids. Abnormalities in magnesium also affect other electrolytes such as sodium, calcium, and potassium, so early detection and treatment is recommended (1). Hypermagnesemia is a rare electrolyte abnormality which occurs in less than 1% of the general population (2), but is found in 7–9% of hospitalized patients with a severe disease (3,4). Recently, it has been reported that even mild hypermagnesemia is correlated with pneumonia in patients with severe diseases (4,5); this suggests that hypermagnesemia should be avoided to minimize suffering, such as dyspnea, even in end-stage cancer patients who are receiving palliative care. Another study has shown that hypermagnesemia is even more prevalent in palliative care cases—occurring in 13% of patients—and is correlated with poor prognosis in end-stage cancer patients (6). Thus, little effort is being made in daily clinical practice to measure serum magnesium levels and avoid hypermagnesemia, including in palliative care, despite the fact that hypermagnesemia can lead to a variety of disorders. This is because avoiding previously identified risk factors can easily prevent injury from hypermagnesemia without measuring serum magnesium levels (7-12). However, an extensive literature review revealed that no studies have examined risk factors for hypermagnesemia with regard to end-stage cancer patients receiving palliative care.
Therefore, we designed a cross-sectional study aimed at identifying risk factors of hypermagnesemia specific to end-stage cancer patients receiving palliative care. After logistic regression analysis was used to investigate previously identified risk factors of hypermagnesemia and physical conditions specific to end-stage cancer patients, we then considered whether these risk factors could be avoided in end-stage cancer patients. We present the following article in accordance with the STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) reporting checklist (available at http://dx.doi.org/10.21037/apm-20-986).
Methods
Study design
This is a cross-sectional study.
Sample size calculation
To investigate risk factors for hypermagnesemia in end-stage cancer patients receiving palliative care in a palliative care unit, we used logistic regression for seven already-known risk factors that we considered likely to be observed in a palliative care unit: Renal dysfunction, magnesium oxide laxative use, active vitamin D use, constipation, hypothyroidism, and lithium use (7-12). Renal dysfunction was defined by chronic kidney disease (CKD) grade 3 or higher. Constipation was defined by the Roma IV classification (13). Hypothyroidism was defined as low free thyroxine or high thyroid-stimulating hormone (TSH). Because different cut-off values of oral magnesium oxide laxative doses for the development of hypermagnesemia have been previously reported (11,12), we performed a preliminary survey in September and October 2019, and the cut-off value was calculated by the receiver operating characteristics (ROC) curve method. The cut-off value (area under the ROC curve: AUC) of oral magnesium oxide laxative dose was 330 mg/day (0.67). Because the minimum dose of oral magnesium oxide laxative in our hospital is 330 mg/day, we did not set a cut-off value in this study, and the presence or absence of oral magnesium oxide laxative was considered. Reference values were determined based on the common reference ranges established by the Japanese Committee for Clinical Laboratory Standards (JCCLS) (14). For parameters not described by the JCCLS, the subject hospital’s reference ranges were used. In addition, to determine whether end-stage cancer patient status is a risk factor for hypermagnesemia, the following two factors were considered for logical regression analysis: physical status and prognosis. Physical status was evaluated using the Eastern Cooperative Oncology Group Performance Status (ECOG PS). Prognosis was assessed using the Palliative Prognostic Score (PaP Score), where a score above 11 points reflected a <30% probability of 30-day survival (15). Finally, we submitted age and gender to the logical regression analysis to correct for confounding factors. For logical regression analysis of the above 11 factors, more than 110 patients with hypermagnesemia were needed. Since our preliminary survey, performed during September and October 2019, demonstrated that hypermagnesemia was observed in five of 29 patients (17.24%), we calculated that enrolling 639 patients would result in finding 110 patients with hypermagnesemia. To avoid a selection bias in which seasonal dietary changes may alter magnesium intake, we planned to enroll all patients throughout the year to recruit a sufficient number of cases for the calculated sample size. Since our palliative care unit receives about 200–250 patients per year, patients admitted to our palliative care unit in the last 3 years were enrolled in the present study.
Target patients
According to the sample size calculation, all patients with end-stage cancer who were hospitalized in the palliative care unit of Himeji St. Mary’s Hospital, Japan, between January 2017 and December 2019 were enrolled in the present study.
Ethical concerns
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Himeji St. Mary’s Hospital, Japan (No. 020-01) on January 7, 2020. The patients enrolled in the study, or their legal representatives, were given the opportunity to opt out from having their data used in the research during the period of January 9 to February 29, 2020. Information on the opportunity to opt out was presented on the hospital website. The requirement of informed consent was waived by the institutional ethics board.
Clinical data collection
Clinical data were collected from the medical records between February 1 and February 29, 2020. Since there was no JCCLS reference range for serum magnesium levels, the hospital’s reference values (1.8–2.5 mg/dL) were used. In patients where hypermagnesemia was detected several times, clinical data were collected at the first diagnosis. In patients whose blood was collected several times but who were never diagnosed with hypermagnesemia, clinical data were collected when the highest level of serum magnesium was observed.
Statistical analysis
Logistic regression analysis was performed using EZR in R commander version 1.37 (16). All analyses were two-sided, and the statistical significance was set at 0.05. Since the present study is a cross-sectional study that extracts data from the medical records of patients already out of practice, a deficit in outcome (presence or absence of hypermagnesemia) due to a lack of measurement was assumed. Therefore, as a sensitivity analysis, we performed a logistic regression analysis identical to the main analysis, albeit at an extreme setting, assuming that all cases in which serum magnesium levels were not measured had hypermagnesemia or did not have hypermagnesemia.
Results
Incidence of hypermagnesemia
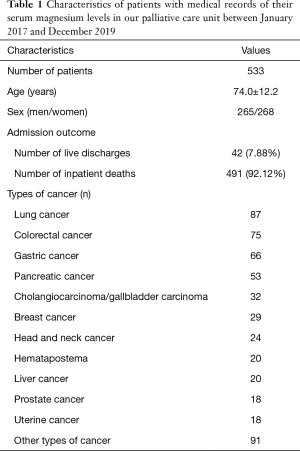
During the study period, 674 patients with end-stage cancer were hospitalized in the hospital’s palliative care unit. These patients were all Japanese. Among them, we were able to obtain 544 blood samples and measure the serum magnesium levels of 533 patients; 42 of the 533 patients were discharged alive, and their final outcome could not be tracked. Table 1 presents the characteristics of patients with a medical record of their serum magnesium levels. Hypermagnesemia was observed in 123 patients (23.08%).
Full table
Risk factors of hypermagnesemia
Lithium use and hypothyroidism were excluded from the logical regression analysis for the following reasons. Since only one patient was using lithium, the number of cases was considered insufficient for statistical processing. There were 12 patients who were taking Levothyroxine, but none of them had thyroxine and TSH levels measured, so we determined that none of them could be accurately diagnosed with hypothyroidism. A logistic regression analysis was performed using renal dysfunction, constipation, oral magnesium oxide laxatives, oral active vitamin D3 preparations, hypercalcemia, ECOG PS, PaP score, age, and sex. The results revealed that the predictors of a significantly higher rate of hypermagnesemia were: renal dysfunction, short prognosis prediction (PaP Score >11), and oral magnesium oxide laxatives (Table 2).

Full table
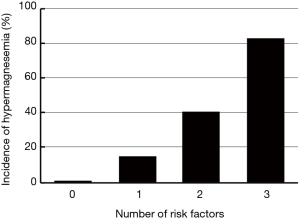
Relationship between the number of risk factors and incidence of hypermagnesemia
Based on the results shown in Table 2, we analyzed the relationship between the number of risk factors of hypermagnesemia and the incidence of hypermagnesemia (Figure 1). The incidence of hypermagnesemia in each group, according to their number of risk factors, was 0.82% (1/122) in the group with no risk factors; 14.61% (32/219) in the group with one risk factor; 40.49% (66/163) in the group with two risk factors; and 82.76% (24/29) in the group with three risk factors.
Sensitivity analysis
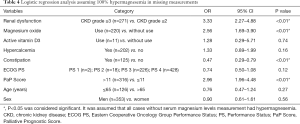
A significantly higher predictor of hypermagnesemia was precisely consistent with the main result, assuming that all the cases without serum magnesium levels measurement did not have hypermagnesemia (Table 3). On the other hand, assuming that all of the cases without serum magnesium levels measurement had hypermagnesemia, constipation was detected as a significantly lower predictor of hypermagnesemia in addition to the three factors indicated in the main results (Table 4).

Full table
Full table
Discussion
Incidence of hypermagnesemia
The incidence of hypermagnesemia in the present study (23.08%) seems to be higher than that of hospitalized patients who received palliative care in Egypt, as reported by Alsirafy et al. (6). However, inpatient death rates significantly differed between the two studies (91.12%, current study vs. 41.7%, Alsirafy et al.), suggesting that the overall medical condition of the present study’s target patients was significantly different. Indeed, when participants were limited to those who died as inpatients, the incidence of hypermagnesemia in Alsirafy et al.’s study (19.4%) was very similar to our findings (6). These results indicate that the incidence of hypermagnesemia in end-stage cancer patients is around 20%, which is higher than that of outpatients and inpatients who are hospitalized for other diseases (2-4).
Risk factors of hypermagnesemia
This study revealed that the significant predictor variables of hypermagnesemia are renal dysfunction, short prognosis prediction (PaP Score >11), and oral administration of magnesium oxide laxatives. The result of sensitivity analysis, assuming no hypermagnesemia in all cases where serum magnesium levels were not measured, is consistent with the main result (Table 3). On the other hand, the result of the sensitivity analysis, assuming hypermagnesemia in all cases where serum magnesium levels were not measured, was not consistent with the main result, and shows that constipation is a significantly lower predictor of hypermagnesemia (Table 4). This result contradicts previous reports that constipation is a risk factor for hypermagnesemia (7-12). Because the incidence of hypermagnesemia was 23.08% in cases where serum magnesium levels were measured, we determined that assuming hypermagnesemia in all cases where serum magnesium levels were not measured was a more extreme setting, compared with assuming no hypermagnesemia in all cases where serum magnesium levels were not measured. Even in a more extreme setting, the result of sensitivity analysis was consistent with the main result with the exception of constipation. This suggests that this result was fairly robust.
Renal dysfunction and oral magnesium oxide laxatives have already been reported as risk factors of hypermagnesemia (7-12). Oral magnesium oxide laxatives increase serum magnesium levels due to the magnesium intake, while renal dysfunction increases serum magnesium levels due to decreased magnesium excretion. It should be noted, however, that a short prognosis prediction has not previously been reported as a risk factor of hypermagnesemia; this is a new finding of the present study. Since there are no previous findings on the matter, we do not believe that a short prognosis prediction is directly related to magnesium intake or excretion. On the other hand, it is well known that muscle mass decreases in end-of-life cancer patients (17). Loss of muscle mass results in lower serum creatinine levels, and the estimated glomerular filtration rate is calculated to be higher than real renal function. Many patients predicted to have a short prognosis may have had renal dysfunction and decreased magnesium excretion, even though their estimated glomerular filtration rate has not declined.
Relationship between the number of risk factors and incidence of hypermagnesemia
This study revealed that the incidence of hypermagnesemia increased as the prevalence of risk factors increased. This suggests that it is important not to use magnesium oxide laxatives on end-stage cancer patients when renal dysfunction is observed and short prognosis is predicted (e.g., by having a PaP Score above 11 points). By avoiding magnesium oxide laxatives, hypermagnesemia may be prevented.
Study limitations
This cross-sectional study has some limitations. First, it failed to evaluate some rare genetic diseases, such as familial hypercalciuric hypercalcemia, which were determined to be risk factors of hypermagnesemia by earlier studies. However, since these diseases are by definition rare, testing for them would not have had a significant impact on the results of the present study. Second, we did not measure the serum levels of parathyroid hormone and parathyroid hormone-related peptides in patients with observed hypercalcemia of the risk factors of hypermagnesemia; thus, we cannot ensure that these patients were accurately diagnosed. Finally, all the participants were recruited from a single center; thus, they could not represent the general population of end-stage cancer patients admitted to palliative care units, and the results must be interpreted with caution.
Conclusions
Risk factors for hypermagnesemia in end-stage cancer patients were renal dysfunction, short prognosis, such as through a PaP Score of above 11 points, and magnesium oxide laxative use. To prevent hypermagnesemia, it is important not to use magnesium oxide laxatives on end-stage cancer patients when renal dysfunction is observed and a short prognosis is predicted. Other multicenter studies are needed to validate the results of this study.
Acknowledgments
We would like to thank Editage (www.editage.com) for their English language editing services.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-986
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-986
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-986). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Himeji St. Mary’s Hospital, Japan (No. 020-01) on January 7, 2020. The patients enrolled in the study, or their legal representatives, were given the opportunity to opt out from having their data used in the research during the period of January 9 to February 29, 2020. Information on the opportunity to opt out was presented on the hospital website. The requirement of informed consent was waived by the institutional ethics board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cascella M, Vaqar S. Hypermagnesemia. StatPearls, 2020. Available online: [Cited 2020 July 9].https://www.ncbi.nlm.nih.gov/books/NBK549811/#article-23189.s1
- Schimatschek HF, Rempis R. Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res 2001;14:283-90. [PubMed]
- Hashizume N, Mori M. An analysis of hypermagnesemia and hypomagnesemia. Jpn J Med 1990;29:368-72. [Crossref] [PubMed]
- Thongprayoon C, Cheungpasitporn W, Srivali N, et al. Admission serum magnesium levels and the risk of acute respiratory failure. Int J Clin Pract 2015;69:1303-8. [Crossref] [PubMed]
- Nasser R, Naffaa ME, Mashiach T, et al. The association between serum magnesium levels and community-acquired pneumonia 30-day mortality. BMC Infect Dis 2018;18:698. [Crossref] [PubMed]
- Alsirafy SA, Sroor MY, Al-Shahri MZ. Predictive impact of electrolyte abnormalities on the admission outcome and survival of palliative care cancer referrals. J Palliat Med 2009;12:177-80. [Crossref] [PubMed]
- Yu ASL, Gupta A. Hypermagnesemia: causes, symptoms, and treatment. Goldfarb S (ed). UpToDate Inc., Massachusetts, 2019. Available online: [Cited 2020 March 2].https://www.uptodate.com/contents/hypermagnesemia-causes-symptoms-and-treatment?search=6.%09Alan%20S%20L%20Yu,%20Aditi%20Gupta:%20Hypermagnesemia:%20Causes,%20symptoms,%20and%20&source=search_result&selectedTitle=1~128&usage_type=default&display_rank=1
- Van Laecke S. Hypomagnesemia and hypermagnesemia. Acta Clin Belg 2019;74:41-7. [Crossref] [PubMed]
- Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012;5:i3-i14. [Crossref] [PubMed]
- Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem 2014;51:179-88. [Crossref] [PubMed]
- Wakai E, Ikemura K, Sugimoto H, et al. Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: a retrospective cohort study. J Pharm Health Care Sci 2019;5:4. [Crossref] [PubMed]
- Mori H, Suzuki H, Hirai Y, et al. Clinical features of hypermagnesemia in patients with functional constipation taking daily magnesium oxide. J Clin Biochem Nutr 2019;65:76-81. [Crossref] [PubMed]
- Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [Crossref] [PubMed]
- Ichihara K, Yomamoto Y, Hotta T, et al. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann Clin Biochem 2016;53:347-56. [Crossref] [PubMed]
- Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage 1999;17:231-9. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Wallengren O, Iresjö BM, Lundholm K, et al. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer 2015;23:79-86. [Crossref] [PubMed]