
Changes in circulating follicular helper T cells in peripheral blood of patients with acute hepatitis C virus infection
Introduction
Acute hepatitis C is a form of early hepatitis C infection, primarily transmitted by blood and presents a significant burden to the health of sufferers. In patients with acute HCV infection, only ~15% of patients can achieve curity spontaneously without anti-viral treatment, and the other., 85% of patients develop chronic HCV infection. Some patients develop more serious diseases, such as hepatic cirrhosis. Currently, the alanine aminotransferase (ALT), hepatitis C virus antibody (anti-HCV) and hepatitis C virus ribonucleic acid (HCV RNA) are utilized in the diagnosis of acute hepatitis C infection and the monitoring of the progress and the therapeutic efficacy of acute hepatitis C infection.
Previous studies have shown that classical immune cells, such as CD8 + T cells, play an important role in acute and chronic HCV infection (1-3). However, there is still a lack of research on some newly emerging immune cell populations. T follicular helper cells (Tfh) are a newly discovered group of immune cells, located in lymphoid follicles. Their main function is to regulate antibody production, generated by B cells. However, the research progress of Tfh cells in clinical diseases is very slow, due to the difficulties in obtaining samples of lymphoid follicles in human tissues (3,4). In 2011, Morita et al. demonstrated the functional equivalence of CD4 + CXCR5 + Tfh cells in peripheral blood (cTfh) with Tfh cells in lymphoid follicles. Thus way, it provides a new and viable way to study acute HCV infection by investigating the changes of cTfh cells in peripheral blood.
Previous studies demonstrate the involvement of cTfh in chronic HCV infection (5-10). However, few studies have reported the role of cTfh cells in acute HCV infection. This study further clarifies the changes of cTfh before and after the anti-viral therapy. The results of this study will help to provide important theoretical and practical significance for clinical exploration of more effective treatment methods.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1179).
Methods
Study subjects
A total of 105 subjects were enrolled in this study. Subjects were divided into three groups, including 35 healthy people, 35 patients with acute HCV infection and 35 acute HCV patients with antiviral therapy. patients with acute hepatitis C infection were selected from the Digestive Department of Affiliated Hospital of Jiangnan University from August 2018 to May 2019, 35, including 48 males and 22 females, aged 25–66 years. All the acute HCV patients were in accordance to the diagnostic criteria for acute HCV infection as follows: history of blood transfusion, history of blood products or a history of exposure to hepatitis C virus. Clinical manifestations: general fatigue, nausea and right-quarter rib pain, mild hepatomegaly, splenomegaly in some patients, etc. Laboratory tests: The serum anti-HCV and HCV RNA were positive and the serum ALT value was more than 10 times higher than that of normal persons, or the serum anti-HCV was negative, while the HCV RNA test was positive
In the control group, 35 health examinees were selected at the same period, including 24 males and 11 females, aged 20–66 years. All the healthy subjects had no history of other diseases, and had no abnormal indexes in liver imaging and serology.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Affiliated Hospital of Jiangnan University. All participants in the study signed the informed consent form.
Detection of surface-related molecules in cTfh by flow cytometry
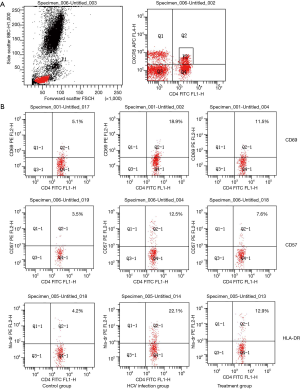
CD4+CXCR5+T cells with CD69+/CD57+/HLA-DR+ were identified as cTfh. Surface-related molecules were detected with BD PharmingenTM Flow Kit.Whole blood was collected from participants of all three groups. One hundred µL of each anticoagulant blood sample were added to respective flow sampling tubes. Anti-CD4 FITC (5 µL), CXCR5 ALEXA 647 and CD69 PE were added prior to incubating at room temperature for 15 minutes. Hemolysin (2 mL) was added before incubation at room temperature for 15 minutes; PBS (2 mL, PH7.2) was added prior to centrifugation at 800 xg for 5 minutes. The supernatant was subsequently isolated. Approximately 500 µL PBS was added to the supernatant prior to detection via flow cytometry. Preparation of samples for CD57 and HLA-DR detection were performed using the same method as CD69. Data analysis was conducted with (BD PharmingenTM) software.
Cytokine expression by ELISA Kit
Venous blood collected from hepatitis C infection, treatment and control groups were stored at room temperature for 10–20 minutes to allow for coagulation. Samples subsequently underwent centrifugation for approximately 10 minutes (1,000 xg). The supernatant should be carefully collected and tested as soon as after extraction. If the test can not be carried out immediately, the specimens can be stored at −20 °C for examination. The test was performed in accordance with manufacturer’s instructions. The regression equation of the standard curve was obtained with ELISA Calc using the concentration and OD value. The logistic curve (four parameters) was used to fit the model, prior to determining cytokine expression levels in each group.
Analysis of correlation between cTfh ratio and HCV RNA content in hepatitis C infection group and treatment group
Pearson correlation analysis was used to analyze the relationship between cTfh and HCV RNA content in the infected group, expressed as a proportion. This provides the theoretical basis for evaluating treatment efficacies for acute hepatitis C infection.
Statistical processing
All experimental data were processed and analyzed by IBM® SPSS23 statistical software. The statistical data is expressed as mean ± standard deviation (
Results
Detection of surface-related molecular markers of cTfh by flow cytometry
Figure 1 illustrates the results of flow cytometry. The percentage of CD69 in the acute HCV infection group was (18.90%±9.29%) higher than the control group (5.10%±4.21%) and antiviral treatment group (11.50%±5.38%). The percentage of cTfh cells decreased significantly after antiviral treatment, but remained higher than the control group (P<0.05).
Detection of cytokine expression by ELISA Kit
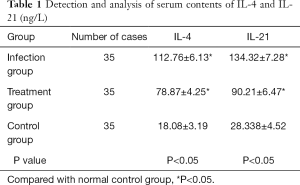
The results of cytokine detection are shown in Table 1. ELISA detection of cytokine expression by cTfh cells in acute HCV infection, treatment and control groups showed significantly higher levels of IL-4 and IL-21 in acute HCV infection group compared to the treatment and control groups. Levels of IL-4 and IL-21 decreased significantly, post treatment but they remained higher than those of the control group.
Full table
CD4+CXCR5+cTfh ratio and HCV RNA content in infected group
Pearson correlation analysis showed that the proportion of cTfh (%) in infected group was negatively correlated with HCV RNA content (Log10) (r=−0.6858, P=0.0028). The results are shown Figure 2.

Discussion
Acute hepatitis C in adults is an infectious disease, mediated by HCV that causes a significant burden to the patients’ health. Studies have shown that the pathogenesis of HCV infection mainly involves immune mediation and direct damage caused by HCV, which are divided into viral and host factors. Viral factors include viral replication ability, genotype, immunogenicity of viral polypeptide. Host factors encompass innate and cellular immune responses, along with humoral immunity.
Previous studies establish Th1-specific cytokines in cellular immunity as vital in controlling acute HCV infection (11-13). Recent international immunological research shows that cTfh plays a key role in the pathogenic factors of chronic HCV infection. However, little is known about the changes associated with cTfh cells in acute HCV infection. This study analyzes the role of cTfh cells in the pathologic process of acute HCV infection, providing new information to aid in novel discoveries.
Ctfh cells are a newly identified group of immune cells (13) which play a key role in many viral-related infection mechanisms (9). These cells are fast becoming a focus in the study of hepatitis infections. They are characterized by the presence of CXCR5, CD69, HLA-DR, CD57 chemokine receptors and secrete cytokines IL-21 and IL-4, which play an important role in the development and formation of cTfh cells. The results of Spaan et al. (14) showed that the expression of ICOS + Tfh cells and cytokine IL-21 increased significantly in the course of acute HCV infection, suggesting that cTfh cells play a key role in the regulation of immune response to acute HCV infection. Alternatively, IL-21 receptors are widely distributed in B cells and cTfh cells, which can positively promote the expression of CXCR5 in patients with acute hepatitis C through autocrine signaling (9,15,16).
The results of this study showed that the proportion of CD69, CD57 and HLA-DR in the acute hepatitis C infection group was significantly higher than the control and the antiviral treatment groups. After antiviral treatment, the proportion of cTfh cells decreased significantly, but remained higher than the control group. This indicates that antiviral treatment was effective. The hepatitis C infection group showed greater cytokine release, with higher Serum IL-4 than that in the control group. The contents of IL-21 and IL-4 in the treatment group and the control group decreased significantly after antiviral treatment, but remained higher than those in the control group. These results demonstrate that cTfh cells and cytokines secreted by cTfh cells play an important role in the process of eliminating acute HCV in the body. Regulating the proportion of related markers of cTfh cells will play an important role in the treatment of the disease.
There were some limitations in the current study. Firstly, the case size enroll in this study was small. Secondly, the level of IL-21 in cTfh cells were not be detected directly. Thirdly, the follow-up data was lacking in this study. Lastly, the underlying mechanism has not been well explored. All these may lead to the statistical bias of the results.
In conclusion, from an acute HCV infection standpoint, this study further clarified the specific pathogenesis of CD69, CD57, HLA-DR and its secreted cytokines IL-4, IL-21 in acute hepatitis C, providing an important foundation for further pre-clinical studies and more effective clinical exploration. This provides theoretical and practical basis for clinical treatment of acute hepatitis C infectious diseases.
Acknowledgments
Funding: Scientific Research Projects of Wuxi Health Planning Commission (MS201813).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1179
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1179
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1179). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants in the study signed the informed consent form. This study was approved by the Ethics Committee of Affiliated Hospital of Jiangnan University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tao JH, Cheng M, Tang JP, et al. Foxp3, Regulatory T Cell, and Autoimmune Diseases. Inflammation 2017;40:328-39. [Crossref] [PubMed]
- Lee MH. Risk of hepatocellular carcinoma for patients treated with direct-acting antivirals: steps after hepatitis C virus eradication to achieve elimination. Transl Gastroenterol Hepatol 2018;3:15. [Crossref] [PubMed]
- Zhang J, Liu W, Wen B, et al. Circulating CXCR3+ Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci Rep 2019;12;9:10090.
- Wrensch F, Ligat G, Heydmann L, et al. Interferon-Induced Transmembrane Proteins Mediate Viral Evasion in Acute and Chronic Hepatitis C Virus Infection. Hepatology 2019;70:1506-20. [Crossref] [PubMed]
- Gaeta GB, Puoti M, Coppola N, et al. Treatment of acute hepatitis C: recommendations from an expert panel of the Italian Society of Infectious and Tropical Diseases. Infection 2018;46:183-8. [Crossref] [PubMed]
- Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011;34:108-21. [Crossref] [PubMed]
- Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000;192:1553-62. [Crossref] [PubMed]
- Fang X, Tong Y, Tian H, et al. Rapid de novo generation of antigen specific human B cells with expression of Blimp-1 and AID by in vitro immunization. Exp Cell Res 2017;352:53-62. [Crossref] [PubMed]
- Raziorrouh B, Sacher K, Tawar RG, et al. Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology 2016;150:696-706.e3. [Crossref] [PubMed]
- Zhang J, Liu W, Xie T, et al. Elevated LAG-3 on CD4+ T cells negatively correlates with neutralizing antibody response during HCV infection. Immunol Lett 2019;212:46-52. [Crossref] [PubMed]
- Girometti N, Devitt E, Phillips J, et al. High rates of unprotected anal sex and use of generic direct-acting antivirals in a cohort of MSM with acute HCV infection. J Viral Hepat 2019;26:627-34. [Crossref] [PubMed]
- Ackermann C, Smits M, WoostR, et al. HCV-specific CD4+ T cells of patients with acute and chronic HCV infection display high expression of TIGIT and other co-inhibitory molecules. Sci Rep 2019;23;9:10624.
- Weinstein JS, Herman EI, Lainez B, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016;17:1197-205. [Crossref] [PubMed]
- Spaan M, Kreefft K, de Graav GN, et al. CD4+ CXCR5+ T cells in chronic HCV infection produce less IL-21, yet are efficient at supporting B cell responses. J Hepatol 2015;62:303-10. [Crossref] [PubMed]
- Wen B, Zhang J, Liu W, et al. HBV coinfection with HCV alters circulating Tfh cell distribution and impairs HCV neutralizing antibody responses. J Viral Hepat 2019;26:1002-10. [Crossref] [PubMed]
- Abe K, Takahashi A, Imaizumi H, et al. Interleukin-21 plays a critical role in the pathogenesis and severity of type I autoimmune hepatitis. Springerplus 2016;18;5:777.
(English Language Editor: E. Tan)


