
Surgical treatment of spinal thoracic metastases with nerve injury in patients with moderate-to-severe spinal cord injury
Introduction
The spine is one of the most common sites of spread in metastatic cancer, ranking third as a metastatic site of malignant tumors, second only to the lung and liver. Relevant epidemiological data indicate that about 5–30% of patients with metastatic cancer in the clinic may be associated with spinal involvement (1), which is one of the main causes of poor quality of life (QOL) and death of patients with malignant tumors (1,2). Cancerous lesions in patients with spinal metastases often invade the vertebral body and spinal canal. When the patient has poor spinal stability, fractures can easily occur, which in turn causes epidural spinal cord compression (ESCC), leading to neurological deficits (3). These patients often face the risk of paraplegia. If they are not treated in time, they will eventually experience paraplegia, incontinence, tract infections, cumulus pneumonia, skin pressure ulcers, and other complications, which accelerate the death of patients (3). In addition, patients are often accompanied by cancer pain of varying degrees, which is extremely severe and has a serious impact on the patient’s QOL. Effective treatment to clear tumor lesions, save spinal cord function, relieve cancer pain, prolong the survival time of patients, and improve the QOL has always been the focus of clinical research.
At present, there is no satisfying treatment that can prolong the life expectancy of patients with spinal metastases. The main interventions are still stereotactic radiotherapy and surgery. Because a variety of tumors are relatively sensitive to radiation, stereotactic body radiotherapy (SBRT) plays an important role in the treatment of spinal metastases. This therapy belongs to the category of precision radiotherapy, which has many advantages including safety, few complications, light adverse reactions, and high rate of tumor local diffusion control. Its efficacy in pain relief and palliative treatment to improve the QOL and prolong the expected survival time has been clinically recognized (4). However, radiotherapy has certain limitations, including damage to the spinal cord, especially for patients who already have symptoms of spinal cord compression. Therefore, studies have shown that spinal cord compression may be one of the drawbacks of stereotherapy, and implementing radiotherapy without appropriate consideration may not benefit patients (5).
In recent years, surgery has been proven to be a feasible treatment option for such patients. Presently, the commonly used surgical methods are total en bloc spondylectomy (TES), vertebroplasty, internal fixation, and fusion. However, these operations have certain limitations. For example, TES surgery is difficult to implement (6), while the latter two procedures have no significant advantages over other operations in terms of spinal nerve function protection, improving QOL, and prolonging survival (7). Some investigators have proposed the concept of spinal tumor isolation surgery, which has received widespread clinical attention (8). This concept is used to separate spinal metastases from the dura mater during operation, which not only helps to save the spinal cord function of patients with metastatic spinal tumors, but also relieves the symptoms of spinal cord compression. Furthermore, the gap around the spinal cord provided by this approach more optimally lays the ground for the next step of high-intensity stereotactic radiotherapy (9).
Conventional laminectomy can cause decompression to a certain extent. However, because the tumor often invades the surface of the dura mater, the separation must be performed during the operation. Because of this, the tumor is not always completely separated, resulting in insufficient decompression. At the same time, if the separation of the posterior spinal cord is not complete, it will reduce the efficacy of later radiotherapy and other related treatments and accelerate relapse (10,11). Progress in treatment paradigms has been made significantly over the past decade. Incorporating stereotactic radiosurgery into these treatments has been particularly effective and safe. Subdural decompression surgery with an epidural incision performed under a microscope can reduce the compression of tumor tissue and reduce the tension of the dura mater, fully decompress the spinal cord, and promote the recovery of spinal cord injury. The purpose of this study was to explore the surgical strategy, safety, and effectiveness of nerve rescue in patients with spinal thoracic metastases with moderate-to-severe spinal cord injury. We specifically evaluate the use of epidural incision combined with decompression of total laminectomy and decompression in conventional lesions. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1507).
Methods
Study population
This was a historically controlled study. According to the inclusion and exclusion criteria, from June 2016 to June 2018, a total of 42 patients with MRI-confirmed metastases of the thoracic spine and moderate or severe spinal cord injury who received conventional decompression of laminectomy combined with internal fixation were collected as the control group. A total of 38 patients who underwent conventional decompression of laminectomy combined with durotomy were selected as the observation group. The observation group included 26 males and 12 females, aged 47–74 (60.14±7.29) years old. Primary tumor types included 16 cases of lung cancer, 11 cases of liver cancer, 6 cases of prostate cancer, and 5 with other types. The control group included 27 males and 15 females aged 45–75 (61.20±8.34) years old. Primary tumor types included 18 cases of lung cancer, 12 cases of liver cancer, 5 cases of prostate cancer, and 7 with other types. There was no statistically significant difference in gender, age, or primary tumor type between the two groups (all P>0.05).
The inclusion criteria for patients were the following: (I) age >45 years with thoracic metastatic tumor and spinal cord injury (T1–T12); (II) preoperative magnetic resonance imaging (MRI) showing complete or incomplete spinal cord injury accompanied by a degree of spinal cord compression (ESCC) grade 2–3; (III) a Tomita score and Tokuhashi score indicating the patient could no longer undergo total tumor resection but only palliative decompression surgery; (IV) spinal instability neoplastic score (SINS) suggesting spinal instability; (V) good cardiopulmonary function and ability to tolerate surgery and conservative treatment. Meanwhile, the exclusion criteria were the following: (I) other neurological diseases (stroke, peripheral neuropathy, craniocerebral injury, etc.); (II) spinal tuberculosis, diabetes, severe infectious diseases, accompanied by severe heart, liver, kidney, and other disorders; (III) severe injuries to other parts of the body; (IV) moderate-to-severe cognitive impairment; (V) severe Alzheimer’s disease and Parkinson’s disease that significantly affected walking ability. Patients who volunteered to participate in this study and cooperated with the follow-up work of this study understood and signed informed consent. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of the PLA General Hospital and informed consent was taken from all the patients.
Surgical procedure
All surgeries in this study were performed by the same group of surgeons. The control group underwent general decompression and decompression of vertebral laminectomy. The operation method was performed as follows. After successful anesthesia, the patient was placed in the prone position. Conventional iodine and alcohol were used to sterilize the skin of the surgical field, and a sterile towel sheet was placed over the surgical field. A posterior thoracic spine midline incision was made to sequentially cut the skin, subcutaneous tissue, and thoracolumbar dorsal fascia, and a subperiosteal dissection of the muscles was performed along both sides of the spinous process, revealing the decompression range of the lamina, upper and lower articular processes, and transverse processes. The pedicle screw was placed in the appropriate segment. After intraoperative fluoroscopy, if the screw position was satisfactory, an ultrasonic bone knife and rongeur were used to remove the spinous process and lamina of the diseased vertebrae that the tumor had invaded. The dural sac was then exposed to make the tumor growing along the spinal canal to the far and near end visible. The dural sac was compressed and adhered to the dura, and the nerve root was loosened. After pedicle puncture, the diseased vertebrae were given a fluoroscopy-guided lumbar puncture and injected with bone cement for strengthening. The tumor tissue and dura mater in the spinal canal were carefully separated, and some diseased tissue was retained for pathology. If the tumor had invaded the pedicle on the ventral side, appropriate excision and separation were performed. Bone wax, gelatin sponge, and other materials were used for hemostasis. The double-sided nail rods were connected, the nuts were pretightened, and the horizontal interlocking rods were placed between the rods. The dural sac and nerve root were once again explored for signs of compression, and to ensure spinal cord pulsation was good, and hemostasis had completely stopped. A large amount of normal saline was used to wash the groove of the fixed segment articular process joint. The articular surface was then removed, the cancellous part of the bone was exposed, and the broken bone was trimmed to backfill the bone graft. The artificial spinal membrane was then placed to cover the spinal cord. The gauze of the instrument was checked, a drainage tube was placed, the muscle, fascia, and subcutaneous tissue were sutured layer by layer, and the skin incision was closed. The sterile dressing was placed to cover the incision, and the drainage tube was connected to the sterile drainage bag. In the observation group, after exposing the dura mater, a surgical microscope was used to remove the tumor tissue on the surface of the dura mater using neurosurgical instruments, the outer layer of the dura mater with high tension was cut, and the pressure was fully applied. Pulsation was restored, and the wound was closed after the same fixation fusion surgery. Both groups received adjuvant radiotherapy and chemotherapy in the oncology department 2 weeks after surgery.
Data collection
- The general data of the two groups, including the operation time, intraoperative blood loss, hospital stay, and hospitalization, were recorded during the perioperative period.
- Visual analogue scale (VAS) was assessed during routine follow-up at 3, 6, and 12 months after surgery. The VAS score ranges from 0 to 10 point, with 0 points indicating no pain and 10 points indicating the most unbearable pain (12).
- The patient QOL scale was evaluated at the same follow-up intervals as VAS above. The scale includes 12 items (appetite, mental, sleep, fatigue, pain, family understanding and cooperation, colleague understanding and cooperation, self-cognition of cancer, attitude to treatment, daily life, side effects of treatment, facial expression). Each item is scored from 1 to 5 points for a total score of 60 points, with a higher the score indicating a better the QOL (13).
- The 36-item short-form health survey (SF-36) assessment includes 8 dimensions. Each dimension and the total score are standardized as follows: (standard conversion formula = (actual score in this item − the lowest possible score in the dimension)/(the highest possible score in this item − the lowest score in this item) × 100%. After standardization, each dimension and the total score can range from 0 to 100 points, with a higher score indicating a better health (14).
- American Spinal Injury Association (ASIA) nerve function grading (15) was recorded before surgery and 3 months after surgery.
- The prognosis of the two groups, including postoperative complications, recurrence, and mortality, were recorded.
Statistical analysis
SPSS v.19.0 (IBM SPSS Statistics, USA) was used for statistical analysis. The variables that accorded with a normal distribution are expressed in mean ± standard deviation. The comparison between two independent sample groups was conducted by groups t-test. In terms of composition ratio, a Chi-square test was used for comparison between two independent sample groups; while nonparametric rank-sum test (Mann-Whitney U test) was used for grade data. A P value <0.05 was considered statistically significant.
Results
Perioperative index comparison between the two groups
All patients successfully completed the operation. The incision healed well, and no infection occurred during or after the operation. There was no statistically significant difference in operation time, intraoperative blood loss, average length of hospital stay, and hospitalization cost between the two groups (P>0.05) (Table 1).
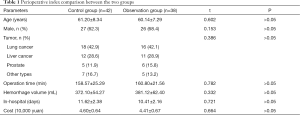
Full table
Comparison of VAS score, QOL scale score, and SF-36 score before and after operation between the two groups
Before surgery, there was no significant difference in VAS score, QOL scale score, and SF-36 score between the two groups (P>0.05). At various time points after follow-up, the VAS scores at 3, 6, and 12 months after operation in the two groups were significantly lower than those before surgery, and the QOL scale score and SF-36 score were significantly higher than those before surgery (P<0.05). Meanwhile, the VAS score at 3, 6, and 12 months after operation for the observation group was significantly lower than that of the control group, and the QOL score and SF-36 score were significantly higher than those of the control group (P<0.05) (Table 2).
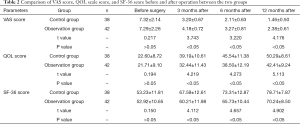
Full table
Comparison of postoperative complications, recurrence rate, and mortality between the two groups
The incidence of postoperative complications in the control group was 19.05% (8/42), while the incidence of postoperative complications in the observation group was 7.89% (3/38). Both groups successfully completed 12 months of follow-up. The recurrence rate of the control group was 14.29% (6/42), and the mortality rate was 26.19% (11/42), while the recurrence rate of the observation group was 5.26% (2/38) and the mortality rate was 18.42% (7/38). Although the postoperative complication rate, recurrence rate, and mortality rate in the observation group were lower than those in the control group, the difference was not statistically significant (P>0.05).
Discussion
The effect of conventional decompression and decompression of vertebral body plus subdural decompression under epidural incision
The results of this study showed that there was no statistically significant difference in the operation time, intraoperative blood loss, average length of hospital stay, and hospitalization cost between the two groups, suggesting the use of epidural incision combined with decompression of total laminectomy and decompression in conventional lesions is effective and safe. Subdural decompression does not increase the operation time, intraoperative blood loss, hospitalization time, or medical cost, and has a high cost-effectiveness. The results of this study showed that the VAS score, QOL score, and SF-36 score of the observation group at 3, 6, and 12 months after operation were better than those of the control group, and the ASIA neurological grade at 3 months after operation was better than that of the control group. These findings indicate that this operation can significantly improve spinal thoracic metastases with moderate to severe spinal cord function, and is more effective in saving nerve function than simple conventional vertebral body decompression. It is also conducive to daily life and QOL improvement. We also found that although the postoperative complications, recurrence rate, and mortality rate of the observation group were lower than those of the control group, these difference were not statistically significant, which may be related to the small sample size included. The sample should thus be expanded in future research in order to confirm the conclusions of the present study.
Analysis of indications, technical advantages, and surgical effects of conventional vertebral body total laminectomy decompression plus epidural incision subdural decompression
In the current state of tumor therapy, there are a variety of treatment methods such as radiotherapy and surgery, along with newly-targeted drug treatments, that have greatly improved the survival of tumor patients. However, some tumors have already metastasized when they are found. The reason for the patient’s treatment are the symptoms caused by the metastasis of other organs. For conventional disease, total laminectomy and decompression have become the main treatment for patients with spinal metastases and spinal cord compression, especially for patients with grade 2–3 spinal cord compression. The operation can separate the tumor from the dura mater to form a space that relieves the compression of the spinal cord, which can not only achieve good local tumor control, but also reduce surgical complications (16). In addition, surgery reduces the body’s tumor-bearing load and improves the responsiveness of the primary tumor of patients to drugs after radiotherapy and chemotherapy, resulting in more effective control of the tumor. In a certain sense, even before radiotherapy treatment occurs, effective surgical operations can achieve a “multiple effects with half the effort” effect, ensuring that patient’s receive the greatest clinical benefit in terms of survival and QOL (4).
At present, the indications for performing surgery on such patients are mainly the following (17,18): (I) progressive increase in nerve function damage; (II) destruction or fracture of spinal lesions in patients, resulting in spinal instability and spinal cord injury; (III) intractable pain, with no obvious effect after treatment with radiotherapy or oral analgesics; (IV) the patient can tolerate surgery, and the expected survival time is greater than 6 months; (V) spinal cord compression caused by spinal tumor invasion of the spinal canal is acute and subacute, and is in a state of continuous exacerbation. At present, in order to save spinal nerve function, clinical emphasis is placed on early surgery, and the 48-hour surgical time limit is often recommended (19). The implementation of active conventional surgical decompression during this period can effectively relieve the symptoms of spinal cord compression and relieve pain. Landmann et al. (20) reported that among patients with spinal metastases undergoing laminectomy decompression therapy, about 68% of patients had significantly improved sphincter function and 88% of patients had pain relief. In contrast, after receiving only simple radiation therapy, only 33% of patients had significantly improved sphincter function and 72% of patients had pain relief. This suggests that the use of decompression surgery before radiotherapy is of great value for the improvement of nerve function.
Although decompression of conventional laminectomy can relieve the compression of the spinal cord to a certain extent and give the spinal cord some room for movement, it often faces the problem of insufficient surgical decompression, such as when the range of decompression of the laminae fails to be greater than spinal edema/when the pressure range is still there, the spinal cord with compressed edema remains at both ends, which leads to the blockage of cerebrospinal fluid and spinal arteriovenous compression, which is not conducive to the restoration of nerve function (21). In addition, due to the differences in operation of different surgeons, the tumor and the dura mater are not completely separated during the operation. If the separation is not complete, the compressed spinal cord tissue of the edema cannot be fully relieved. Therefore, there is still much room for improvement in decompression of conventional laminectomy.
Epidural decompression under a microscope has been widely conducted in our hospital. With the microscope, decompression under the dura mater not only removes the tumor invasion site on the surface of the dura, but also effectively removes the subdural hematoma and bone fragments at the same time. Subdural release is performed to effectively relieve the compression of the spinal arteriovenous and epidural veins, reduce the pressure on the edema and spinal cord, and create space for the recovery of spinal cord function. Relevant basic and clinical studies have shown that the main treatment mechanism of decompression of the dural incision is reflected in the following aspects: (I) it can effectively reduce the pressure in the spinal canal, relieve local tissue edema, and thus reduce the compression of the spinal cord, which is beneficial to restoration of blood-spinal barrier function (17). (II) Spinal cord microcirculation disorder caused by increased pressure in the spinal canal is also known as the “osteofascial syndrome” of the spinal cord (20). Decompression under the membrane can significantly reduce the pressure outside the spinal cord, thereby improving spinal cord microcirculation. (III) Studies have shown that spinal cord injury can be accompanied by a series of secondary inflammatory reactions, including the activation of inflammatory cells such as monocytes, macrophages, and lymphocytes; infiltration and aggregation in local tissues; the release of pro-inflammatory cytokines including interleukin-6 (IL-6), interleukin-β1 (IL-β1), tumor necrosis factor-α (TNF-α); and glial cell proliferation and increase of extracellular matrix expression (22). By reducing pressure, duratomy can reduce inflammation and edema (23,24). (IV) The spinal cord injury can activate the nuclear factor-κB (NF-κB) signal transduction pathway, inducing inflammation and oxidative stress (25). Decompression of the dural incision effectively relieves ischemia and hypoxia of local nerves, reduces oxidative stress, and accelerates the removal of oxygen-free radicals, thereby reducing lipid peroxidation damage, which is conducive to nerve damage repair. (V) Studies have shown that ischemia, hypoxia, inflammation, and oxygen free radical-induced autophagy play a key role in secondary spinal cord injury (26,27). Yang et al. (28) performed early decompression on a rat model of spinal cord injury and found that the decompression of the spinal cord can significantly inhibit the expression of the LC3-II protein and mRNA, and activate the rapamycin target. The signaling pathway of protein 1 eventually inhibits autophagy and exerts a neuroprotective effect. After metastasis of the thoracic spine tumor, the tumor grows rapidly, the space of the thoracic spinal canal shrinks, and spinal nerve injury progresses rapidly. Therefore, for patients with thoracic vertebral tumor metastasis, more timely surgery is needed to help control the further progression of the disease. The possible adverse reaction of this method involved spine injury which can be avoided by careful operation.
Limitations of the study
This is a historically controlled study and not a prospective case-control cohort study. Therefore, in future clinical practice, a prospective case-control study is needed to confirm the conclusion of the present research. In addition, the included sample size is relatively small and characteristic of a single-center study: there might have been selection bias. It is necessary to expand the sample size for multi-center clinical research in the future. In conclusion, this experiment initially confirmed that patients with spinal cord injury and neurological dysfunction caused by spinal tumor who undergo early subdural open decompression surgery based on routine decompression and decompression of the total laminectomy, can recover neurological function. The recurrence rate and QOL achieved by this approach are superior to those of simple epidural decompression, and thus this technique can prevent spinal cord injury caused by spinal tumors in the elderly. In conclusion, we recommend this strategy in clinical practice for the treatment of spine metastasis.
Acknowledgments
Funding: This study was supported by 2017 Health Special Scientific Research Project (17BJZ44).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1507
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1507
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1507). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of the PLA General Hospital and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Choy WJ, Phan K, Mobbs RJ. Editorial on the integrated multidisciplinary algorithm for the management of spinal metastases. Transl Cancer Res 2019;8:S152-S155. [Crossref]
- Versteeg AL, van Tol FR, Lehr AM, et al. Malnutrition in patients who underwent surgery for spinal metastases. Ann Transl Med 2019;7:213. [Crossref] [PubMed]
- Lin JH. Outcome Analysis of Spinal Canal Decompression and Muscle Preservation After Unilateral Approach and Bilateral Decompression of Minimally Invasive Spine Surgery in Degenerative Lumbar Spine Disorders. Spine J 2012;12:S113. [Crossref]
- Le R, Tran JD, Lizaso M, et al. Surgical Intervention vs. Radiation Therapy: The Shifting Paradigm in Treating Metastatic Spinal Disease. Cureus 2018;10:e3406. [Crossref] [PubMed]
- Husain ZA, Sahgal A, De Salles A, et al. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine 2017;27:295-302. [Crossref] [PubMed]
- Inoue G, Imura T, Miyagi M, et al. Total en bloc spondylectomy of the eleventh thoracic vertebra following denosumab therapy for the treatment of a giant cell tumor. Oncol Lett 2017;14:4005-10. [Crossref] [PubMed]
- Hamad A, Vachtsevanos L, Cattell A, et al. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br J Neurosurg 2017;31:526-30. [Crossref] [PubMed]
- Duffy A, Capanu M, Aboualfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Bate BG, Khan NR, Kimball BY, et al. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine 2015;22:409-15. [Crossref] [PubMed]
- Clarke MJ, Molina CA, Fourney DR, et al. Systematic review of the outcomes of surgical treatment of prostate metastases to the spine. Global Spine J 2017;7:460-8. [Crossref] [PubMed]
- Mielke D, Rohde V. Bilateral spinal canal decompression via hemilaminectomy in cervical spondylotic myelopathy. Acta Neurochir (Wien) 2015;157:1813-7. [Crossref] [PubMed]
- Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497-501. [Crossref] [PubMed]
- Peruselli C, Camporesi E, Colombo AM, et al. Quality-of-life assessment in a home care program for advanced cancer patients: A study using the symptom distress scale. J Pain Symptom Manage 1993;8:306-11. [Crossref] [PubMed]
- Tunis SL, Croghan TW, Heilman DK, et al. Reliability, Validity, and Application of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) in Schizophrenic Patients Treated with Olanzapine versus Haloperidol. Med Care 1999;37:678-91. [Crossref] [PubMed]
- El Masry WS, Tsubo M, Katoh S, et al. Validation of the American Spinal Injury Association (ASIA) Motor Score and the National Acute Spinal Cord Injury Study (NASCIS) Motor Score. Spine 1996;21:614-9. [Crossref] [PubMed]
- Choi D, Fox Z, Albert T, et al. Prediction of Quality of Life and Survival After Surgery for Symptomatic Spinal Metastases. Neurosurgery 2015;77:698-708. [Crossref] [PubMed]
- Bakar D, Tanenbaum JE, Phan K, et al. Decompression surgery for spinal metastases: a systematic review. Neurosurg Focus 2016;41:E2. [Crossref] [PubMed]
- Wright E, Ricciardi F, Arts M, et al. Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg 2018;114:e809-e817. [Crossref] [PubMed]
- Shah AA, Paulino Pereira NR, Pedlow FX, et al. Modified En Bloc Spondylectomy for Tumors of the Thoracic and Lumbar Spine: Surgical Technique and Outcomes. J Bone Joint Surg Am 2017;99:1476-84. [Crossref] [PubMed]
- Landmann C, Hünig R, Gratzl O. The role of laminectomy in the combined treatment of metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 1992;24:627-31. [Crossref] [PubMed]
- Klimo P, Thompson CJ, Kestle JRW, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. [Crossref] [PubMed]
- Hussain AK, Vig KS, Cheung ZB, et al. The Impact of Metastatic Spinal Tumor Location on 30-Day Perioperative Mortality and Morbidity After Surgical Decompression. Spine 2018;43:E648-E655. [Crossref] [PubMed]
- Perkins PG, Deane RH. Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury 1988;19:397-401. [Crossref] [PubMed]
- Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 2018;15:541-53. [Crossref] [PubMed]
- Zhang J, Wang H, Zhang C, et al. Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: a randomized, controlled preclinical research. J Orthop Surg Res 2016;11:34. [Crossref] [PubMed]
- Chong S, Shin SH, Yoo H, et al. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients' survival. Spine J 2012;12:1083-92. [Crossref] [PubMed]
- Wang W, Shen H, Xie JJ, et al. Neuroprotective effect of ginseng against spinal cord injury induced oxidative stress and inflammatory responses. Int J Clin Exp Med 2015;8:3514-21. [PubMed]
- Yang D, Li J, Gu R, et al. Myelotomy suppresses autophagic marker LC3-II expression and elevates mTORC1 expression and improves neurological function recovery in rats with spinal cord injury. Neurology Asia 2013;18:401-7.
(English Language Editor: J. Gray)


