Clinical and EEG characteristics analysis of autoimmune encephalitis in children with positive and negative anti-N-methyl-D-aspartate receptor antibodies
Introduction
Autoimmune encephalitis refers to a kind of disease, in which the immune system of the body responds to the antigens and antibodies produced by central nervous system antigens, resulting in central nervous system damage. The etiology may be the interaction of some autoantibodies, active cells, or related factors with proteins and receptors on the surface of neurons in the central nervous system, resulting in changes in synaptic transmission, synaptic plasticity and neuronal excitability, thereby leading to a series of corresponding clinical symptoms. The main clinical manifestations are seizures, cognitive dysfunction, consciousness disorders, mental behavior disorders, autonomic nervous dysfunction and ventilation disorders (1).
There has been a relatively large proportion of patients suspected of autoimmune encephalitis, with negative serologic antibodies. However, few studies have been conducted on the percentage of children who are positive for receptor antibodies (2-6). In children, the most commonly reported autoimmune encephalitis is anti-NMDAR encephalitis. In a large sample study conducted in the United States, 40% of anti-NMDAR encephalitis incidences occurred in childhood or adolescence (7). In another British study with a relatively small sample size, 23% of child patients were under the age of 18 years old (8). In the present clinical diagnosis of autoimmune encephalitis, 35.7% (10/28) of child patients tested positive for anti-NMDA receptor antibody, and no other types of neuron autoantibodies were detected. These present results revealed that apyrexial onset was more likely to occur in the antibody positive group, when compared to the antibody negative group. However, no etiological positive results were found in both groups. Some studies have reported that anti-NMDAR antibody was detected in 30% of herpes simplex encephalitis (9), while few studies reported that Japanese encephalitis could induce anti-NMDAR encephalitis (10). In the present study, one child patient with positive epidemic encephalitis B antibody was detected in the antibody positive group. The second manifestation of encephalopathy occurred after the second recurrence of encephalitis B. Autoimmune encephalitis caused by the secondary immune injury after infection of the Japanese encephalitis virus (JEV) was considered. These results suggest that specific viral infection may induce anti-NMDA receptor antibody encephalitis, which may lead to a potential autoimmune reaction in the brain, thereby leading to the occurrence of immune-mediated second encephalopathy.
Although an increasing number of studies have been conducted on autoantibodies of the nervous system, autoantibodies could not be detected in a relative proportion of child patients with suspected immune-induced central nervous system. Hence, its diagnosis and treatment remain challenging. The data of 28 child patients with autoimmune encephalitis diagnosed in our department within two years and the follow-up results are reported, as follows. We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-484).
Methods
General data
Study subject
A total of 28 child patients with autoimmune encephalitis, which comprised of 19 males and nine females, who were treated in our Hospital from January 2015 to January 2017, were enrolled in the present study. The onset age varied from eight months old to 11 years and four months old. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Qilu Hospital of Shandong University (KYLL-2020(KS)-613). All child guardian had signed the informed consent.
Inclusion criteria
According to the diagnostic criteria for autoimmune encephalitis published by Lancet Neurology in 2016 (4), the diagnosis can be made when the following four criteria are met:
- Acute course (<3 months), loss of working memory (long-term or short-term memory loss), and altered mental status or mental symptoms;
- Other typical symptoms of autoimmune encephalitis (e.g., typical marginal encephalitis, Bickerstaff brainstem encephalitis, and acute disseminated encephalomyelitis) are excluded;
- A lack of well-characterized neuronal antibodies in serum and the cerebrospinal fluid, which meets at least two of the following criteria: (i) the abnormal magnetic resonance imaging (MRI) suggests autoimmune encephalitis; (ii) the amount of cerebrospinal fluid (CSF) cells increased, CSF specific oligoclonal antibody or CSF IgG index increased, or both occurred; (iii) the CSF presented with inflammatory infiltration and excluded other diseases (such as tumors);
- The reasonable exclusion of other diseases, such as cerebral hemorrhage, cerebral infarction, brain tumors, congenital brain hypoplasia and other infection diseases, et al.
Manifestations of encephalopathy in children: A decline or change in level of consciousness, lethargy, and personality changes or behavioral changes, and at least one of the following clinical manifestations would occur: neuropsychiatric symptoms, epilepsy, motor disorders, and cognitive disorders. Other types of encephalitis are excluded.
Study methods
General data
The clinical data of 28 child patients diagnosed with autoimmune encephalitis were analyzed. The short-term data of patients were obtained by telephone follow-up or electroencephalogram (EEG) follow-up.
Electroencephalogram
The NIHON KOHDEN 32-channel VEEG-1200-C system was used for the video EEG monitoring. The 19-lead recording electrodes were placed in accordance to the international 10–20 system, and the electromyography (EMG) on the surface of bilateral deltoid muscles was simultaneously recorded. A 4-hour EEG was recorded for the child patients, including a complete wake-sleep-arousal cycle. Video electroencephalogram (VEEG) monitoring was performed after admission, and was followed up after three months (multiple VEEG monitoring could be performed, as required).
Auxiliary examination
Serum and cerebrospinal fluid antibody tests, brain MRI and routine biochemical examinations were performed for all child patients.
Results
Personal history
One child suffered from postnatal asphyxia complicated with ischemia and hypoxia, one child had postnatal mild hypoxia, three children had growth and development lag behind the normal children of the same age, two children had febrile convulsion, and one child epilepsy.
Clinical course and performance
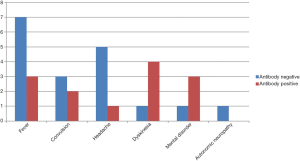
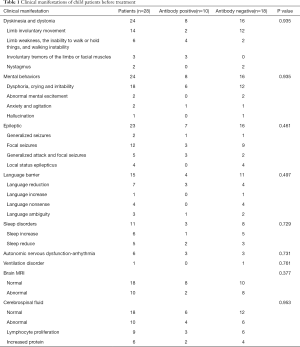
NMDAR-Abs were tested by enzyme linked immunosorbent assay (ELISA). In the antibody positive group, 40% of the child patients had the onset of dyskinesia and dystonia, followed by fever and mental disorder, which accounted for 30%. In the antibody negative group, 39% of child patients had the onset of fever, followed by headache, which accounted for 28%. The clinical manifestations of 28 child patients varied, among which 24 children suffered from dyskinesia and dystonia, including 14 child patients with limb involuntary movement (two child patients in the positive group and 12 child patients in the negative group), six child patients with limb weakness, the inability to walk or hold things, and walking instability (four child patients in the positive group and two child patients in the negative group), three child patients with involuntary tremors of the limbs or facial muscles (positive group), and two child patients with nystagmus (negative group). A total of 24 child patients suffered from mental behaviors, including 18 child patients with dysphoria, crying and irritability (six child patients in the positive group and 12 child patients in the negative group), two child patients with abnormal mental excitement (two child patients in the negative group), two child patients with anxiety and agitation (one child patient in the positive group and negative group, respectively), and one child patient with hallucination (negative group). A total of 23 child patients suffered from epileptic seizures, including two child patients with generalized seizures (one child patient in the positive group and negative group, respectively), 12 child patients with focal seizures (three child patients in the positive group and nine child patients in the negative group), five child patients with the co-existence of generalized attack and focal seizures (three child patients in the positive group and two child patients in the negative group), and four child patients with local status epilepticus (four child patients in the negative group). A total of 15 child patients suffered from language barrier, including seven child patients with language reduction (three child patients in the positive group and four child patients in the negative group), one child patient with language increase (one child patient in the negative group), four child patients with language nonsense (four child patients in the negative group), and three child patients with language ambiguity (one child patient in the positive group and two child patients in the negative group). A total of 11 child patients suffered from sleep disorders, including six child patients with sleep increase (one child patient in the positive group and five child patients in the negative group), five child patients with reduced sleep (two child patients in the positive group and three child patients in the negative group), six child patients with autonomic nervous dysfunction-arrhythmia (three child patients in the positive group and three child patients in the negative group), and one child patient with ventilation disorder (negative group) (Table 1 and Figure 1).
Full table
Imaging and laboratory examinations
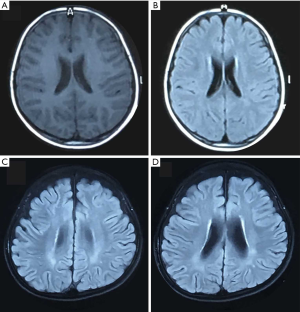
All child patients underwent brain MRI after admission, including 18 normal child patients (eight child patients in the positive group and 10 child patients in the negative group), and 10 abnormal child patients (two child patients in the positive group and eight child patients in the negative group). These mainly included a gray/white matter abnormal signal of the brain, which is common in the frontal, parietal, thalamus and hippocampal region (Figure 2). Furthermore, among these patients, 18 child patients had a normal routine examination of the cerebrospinal fluid (six child patients in the positive group and 12 child patients in the negative group), while 10 child patients had an abnormal routine examination (four child patients in the positive group and six child patients in the negative group). No specificity was found in abnormal patients, with a slight increase in lymphocytes or an increase in protein. Anti-NMDA antibody was detected to be positive in the cerebrospinal fluid of 10 child patients (Table 1).
VEEG monitoring
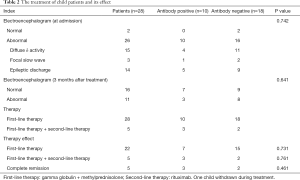
At admission, the results of the 4-hour VEEG in the 28 child patients revealed that 26 child patients (93%) were abnormal, while two child patients (negative group) were normal. In the antibody positive group, four child patients had diffuse δ activity, one child patient had focal slow wave, and five child patients had epileptic discharge. In the antibody negative group, 11 child patients had diffuse δ activity, two child patients had focal slow wave, and nine child patients had epileptic discharge. Epileptic discharge was common in the frontal and anterior temporal regions. Focal seizures began in the frontal, anterior temporal and middle temporal areas, or multifocal areas (Table 2).
Full table
Treatment
After the diagnosis, these child patients received treatment of 1–3 round(s) of methylprednisolone shock and gamma globulin, among which three child patients received one round (two child patients in the positive group and one child patient in the negative group), six child patients received two rounds (one child patient in the positive group and five child patients in the negative group), 14 child patients received three rounds (four child patients in the positive group and 10 child patients in the negative group), and five child patients received additional rituximab treatment for poor effect after hormone shock (three child patients in the positive group and two child patients in the negative group), with oral administration of hormone after shock. One child patient in the negative group was automatically discharged after one course of treatment with hormone shock. Oral antiepileptic drugs were given to child patients with multiple seizures. The oral administration of olanzapine was given to child patients with obvious mental symptoms (Table 2).
Prognosis and follow-up
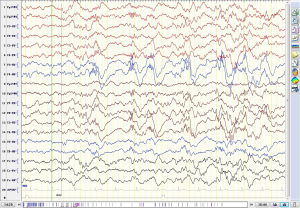
After hospitalization, five child patients basically recovered, and were normally discharged (three child patients in the positive group and two child patients in the negative group), while 22 child patients improved, and were discharged. Among the 22 improved child patients, six child patients continued to suffer from mild mental symptoms, four child patients had mild dyskinesia, two child patients had language dysfunction, one child patient had sleep disorder, and one child patient had mental and motor retardation. The follow-up period ranged within 1–3 years, and the following are the results: 10 child patients completely recovered to normal (four child patients in the positive group and six child patients in the negative group), three child patients had slight limb tremors during sleep or when angry, one child patient had a personality change, one child patient had language reduction, one child patient had mental and motor retardation, eight child patients had sequela of epilepsy, and all of which were focal seizures (one child patient in the positive group and seven child patients in the negative group), and three child patients had relapse (one child patient in the positive group and two child patients in the negative group). The VEEG was reviewed after three months. The background rhythm was normal in 16 child patients (seven child patients in the positive group and nine child patients in the negative group). The background brain wave frequency was mainly 5–8 Hz in eight child patients, including two child patients with diffuse δ activity and one child patient with diffuse low-amplitude fast-wave rhythm release. It is noteworthy that one child patient in the negative group suffered from intractable epilepsy. Focal seizures and local electric seizures were detected by VEEG for many times. At the 23rd month after the onset of the disease, the VEEG monitoring revealed the following: δ brush in the occipital, middle and posterior temporal regions (Table 2, Figure 3).
Discussion
The clinical manifestations of 28 child patients in this group were classified into seven types, including mental symptoms, epileptic seizures, motor disorders, language disorders, sleep disorders, autonomic nervous dysfunction and ventilation disorders. Among these, seven clinical symptoms were found in one child patient in the antibody negative group, while merely one child had numbness and tremor in both lower limbs in the antibody positive group, and one child had fever, headache and dizziness. The treatment results of the immunotherapy were better in these two child patients with mild clinical symptoms. In the antibody negative group, two child patients with prominent mental symptoms were admitted to the Psychiatric Department at an early stage of onset, which emphasized the importance of the early identification of autoimmune encephalitis in children and differentiation from mental diseases. The difference in clinical manifestations was not statistically significant (P>0.05) between the antibody positive group and antibody negative group. Mild and moderate lymphocytes and protein increase were common in the CSF of children with autoimmune encephalitis, and oligoclonal bands could be observed in 50–60% of child patients (11-16). The CSF abnormalities in this group of child patients exhibited a slight increase in cell number, protein increase, and no specificity.
VEEG monitoring is necessary for children with autoimmune encephalitis (17). In this group, four child patients with focal status epilepticus were confirmed by VEEG. Focal seizures were detected in six child patients. Among these, 44 focal seizures were monitored in one child patient, while merely three seizures were recorded by their parents. Furthermore, two focal seizures and four local electric seizures were recorded in one child patient after three months of follow-up, but the parents did not identify the onset of the disease at the same period. The VEEG mainly revealed a diffuse δ slow wave at four hours after admission in this group of child patients, but no δ brush was recorded, which may indicate that the incidence of δ brush was lower in children with autoimmune encephalitis, or that this was correlated to the short recording time of the VEEG. In the antibody negative group, a 5-year-old and 10-month-old child patient was detected by VEEG at the 23rd month after onset (seven years and nine months old), and δ brush was monitored in the bilateral occipital, middle and posterior temporal regions. The 4hVEEG was reviewed several times after the child patient was discharged from the hospital. Focal seizures and/or focal electric seizures were discovered in the origin of the right posterior head in the early drowsiness and non-rapid eye movements (NREM) (I and II) phases. At present, these child patients can go to school, but their intellectual development slightly lags behind normal children of the same age. Schmitt et al. (18) reported that 30% of 23 adult patients with anti-NMDAR encephalitis developed an extreme δ-brushed (EDB) pattern in the early stage of continuous brain function monitoring (CEEG). These patients had a longer hospitalization time, longer CEEG monitoring time, and poorer prognosis. This suggests that the EDB pattern may be a more serious symbol. After active immunotherapy, the EEG gradually improved in at least two patients during hospitalization. Furthermore, in both patients, the regression of EDB was associated with clinical improvement (18). In addition, the study conducted by Wang et al. (19) on children with anti-NMDAR encephalitis revealed that when the δ brush appeared at the peak and trough of δ waves, this indicates a poor prognosis, while when this only occurs in a valley, this indicates a good prognosis (20).
Lancaster (17) considered that seizures in autoimmune encephalitis are correlated to the activity of the disease. Seizures may disappear after other symptoms of autoimmune encephalitis are relieved, and before the treatment of autoimmune encephalitis, seizures are difficult to control with antiepileptic drugs. After one year of follow-up, these seizures disappeared in six child patients, among the 10 children with positive antibody in this group, while one child relapsed and appeared with status epilepticus. The negative antibody was found in 17 patients. Among these patients, 10 patients stopped the use of antiepileptic drugs, and no seizures were found during the follow-up, while seven patients continued to take antiepileptic drugs (one patient suffered from seizures after stopping carbamazepine in-take). All patients had focal seizures.
In the present study, the mortality of anti-NMDA receptor encephalitis was approximately 5%. This shows that 81% of these child patients had a relatively good prognosis after follow-up for two years (12). The recurrence rate of anti-NMDAR encephalitis was not high. Approximately 12% of patients with anti-NMDAR encephalitis relapsed within two years. For autoimmune encephalitis, its recurrence is relatively mild, when compared to the first onset. Usually, merely a single epilepsy or motor disorder is recurrent. Chronic immunosuppressive measures, such as mycophenolate mofetil, azathioprine, or the re-administration of rituximab (7), can be given during relapse. This group of child patients have been followed up for more than one year. Among these patients, 23 child patients (82%) returned to normal or had a mild sequelae, one child patient (4%) suffered from mental and motor retardation (probably related to the primary disease), one child patient (4%) had secondary drug-refractory epilepsy, and three child patients (11%) relapsed. In the antibody positive group, the minimum age was eight months old, and no recurrence was found after three years of follow-up.
The exact mechanism by which nervous system autoantibodies are produced is not known. The two commonly recognized mechanism were pathogen infection and certain tumor tissues, such as herpes simplex virus infection of the central nervous system, which can collectively produce NMDAR antibodies leading to autoimmune encephalitis. Some tumor tissues contain nerve cells or express neuronal proteins, which can also induce the body to produce autoantibodies to attack the nerve or muscle system (21). NMDAR encephalitis was the most common and typical type of autoimmune encephalitis, which tends to develop in young women and children and is closely related to teratomas. However, no tumor was detected in the children with NMDAR encephalitis included in this study. Zhang et al. included 103 children with autoimmune encephalitis, and only one 12-year-old patient with NMDAR encephalitis was diagnosed with ovarian teratoma (22), indicating a low tumor detection rate in children. The pathogenesis was that NMDAR antibody IgG binds to the glutamate N1 subunit of the n-methyl-d-aspartic acid receptor on the surface of neurons, leading to the closure and internalization of NMDAR, resulting in neurological dysfunction. The titer of NMDAR antibody was closely related to the course and prognosis of encephalitis in patients. The higher the titer of antibody, the more serious the disease and the worse the prognosis of patients (23,24). Animal experiments had also confirmed that intraventricular injection of NMDAR antibody in mice leads to decreased number of synaptic NMDAR and memory loss (25). Therefore, NMDAR antibody was not only the core indicator for the diagnosis of NMDAR encephalitis, but also an important biomarker for the progression and prognosis of the disease.
The discovery of autoantibodies on the neuron surface promotes the progress in the study of encephalitis, and provides evidence for clinicians to use immunomodulatory therapy in children. However, it was found that autoantibodies could not be detected in a large proportion of children who were suspected of autoimmune encephalitis. They are similar to child patients with positive anti-NMDAR antibodies in clinical manifestation, EEG characteristics, treatment and prognosis. These child patients may have undetected/unrecognized autoantibodies or other immune mechanisms. The markers for antibody negative diagnosis still need to be further investigated. However, no enough data about the infection test in this study which should be further research in future.
With the increasing understanding of autoimmune encephalitis in China, related reports are gradually increasing. For children suspected with autoimmune encephalitis, serum and cerebrospinal fluid antibody tests, brain MRI and EEG examinations, and systemic tumor screening should be carried out as soon as possible. At the same time, immunotherapy should be carried out as soon as possible, in order to obtain a good prognosis.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding: This study was supported by the Shandong Provincial Natural Science Foundation, China, Grant Number: ZR2016HB38.
Footnote
Reporting Checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-484
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-484
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-484). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Qilu Hospital of Shandong University (KYLL-2020(KS)-613). All child guardian had signed the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune Encephalitis in Children. J Child Neurol 2012;27:1460-9. [PubMed]
- Dutra LA, Abrantes F, Toso FF, et al. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr 2018;76:41-9. [Crossref] [PubMed]
- Koolwal A, Agrawal S, Koolwal GD, et al. Anti-N-methyl-D-aspartate Receptor Encephalitis: Case Series of Psychiatric Presentations. Ann Indian Acad Neurol 2020;23:225-7. [PubMed]
- Dalmau J. Name a brain protein, and an autoantibody shall be found. Neurol Neuroimmunol Neuroinflamm 2015;2:e159. [Crossref]
- Dale RC, Gorman MP, Lim M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol 2017;30:334-44. [Crossref] [PubMed]
- Guo YP, Li XY, Liu HF, et al. linical analysis of 7 cases with anti-Caspr2 antibody-associated autoimmune encephalitis. Zhonghua Yi Xue Za Zhi 2020;100:513-5. [PubMed]
- Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009;66:11-8. [Crossref] [PubMed]
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate anti-body encephalitis:temporal progression of clinical and para-clinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655-67. [Crossref] [PubMed]
- Prüss H, Finke C, Höltje M, et al. Hofmann J N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902-11. [Crossref] [PubMed]
- Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti-N-methyl-D-aspartate receptor encephalitis. J Neurol 2017;264:1127-31. [Crossref] [PubMed]
- Platt MP, Bolding KA, Wayne CR, et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc Natl Acad Sci U S A 2020;117:6708-16. [Crossref] [PubMed]
- Zhang QQ, Zhang YF, Yu N, et al. Differential Diagnosis of Autoimmune Encephalitis from Infectious Lymphocytic Encephalitis by Analysing the Lymphocyte Subsets of Cerebrospinal Fluid. Anal Cell Pathol (Amst) 2019;2019:9684175. [Crossref] [PubMed]
- Zeng Z, Wang C, Wang B, et al. Prediction of neutrophil-to-lymphocyte ratio in the diagnosis and progression of autoimmune encephalitis. Neurosci Lett 2019;694:129-35. [Crossref] [PubMed]
- Kelley BP, Patel SC. Autoimmune Encephalitis: Pathophysiology and Imaging Review of an Overlooked Diagnosis. AJNR Am J Neuroradiol 2017;38:1070-8. [Crossref] [PubMed]
- Brenton JN, Goodkin HP. Antibody-Mediated Autoimmune Encephalitis in Childhood. Pediatr Neurol 2016;60:13-23. [PubMed]
- Armangue T, Titulaer MJ, Málaga I, et al. Spanish Anti-N-methyl-D-Aspartate Receptor (NMDAR) Encephalitis Work Group. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013;162:850-6.e2. [Crossref] [PubMed]
- Lancaster E. The Diagnosis and Treatment of Autoimmune Encephalitis. J Clin Neurol 2016;12:1-13. [Crossref] [PubMed]
- Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: a unique EEG pattern in adults with anti- NMDA receptor encephalitis. Neurology 2012;79:1094-1100. [Crossref] [PubMed]
- Wang J, Wang K, Wu D, et al. Extreme delta brush guides to the diagnosis of anti-NMDAR encephalitis. J Neurol Sci 2015;353:81-3. [Crossref] [PubMed]
- Pillai SC, Hacohen Y, Tantsis E, et al. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics 2015;135:e974-e984. [Crossref] [PubMed]
- Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med 2018;378:840-51. [Crossref] [PubMed]
- Zhang J, Ji T, Chen Q, et al. Pediatric Autoimmune Encephalitis: Case Series From Two Chinese Tertiary Pediatric Neurology Centers. Front Neurol 2019;10:906. [Crossref] [PubMed]
- Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167-77. [Crossref] [PubMed]
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391-404. [Crossref] [PubMed]
- Li Y, Tanaka K, Wang L, et al. Induction of Memory Deficit in Mice with Chronic Exposure to Cerebrospinal Fluid from Patients with Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Tohoku J Exp Med 2015;237:329-38. [Crossref] [PubMed]