
Use of palliative chemotherapy near the end of life: a retrospective cohort study
Introduction
The issue of near-the-end-of-life (EOL) chemotherapy has recently garnered intense research interest. During their last 30 days of life, 13–43% of patients with advanced cancer are treated using chemotherapy (1-3). One of the indicators of aggressive EOL care is chemotherapy within 14 days of death (4). The drugs available and the indications for the use of palliative chemotherapy treatment (PCT) are constantly growing in number; one example of this is the increased use of targeted therapy and immunotherapies. The Health Service Research Committee of the American Society of Clinical Oncology (ASCO) has stated that treatment can be provided if it improves the quality of life of patients with metastatic cancer, even if it does not improve their survival (5). Psychosocial support, decision-making, symptom management, hospice care, and survival are all considered parts of high-quality EOL care (6-8). However, palliative chemotherapy often carries the risk of adverse events that could negatively impact patients without prolonging their survival (9). The decision to provide palliative chemotherapy near the end of a patient’s life involves balancing clinical benefits with potential harm from side effects. In most countries, EOL treatment for patients with advanced cancer has become increasingly aggressive (10), however, the underlying reasons that contribute to the overuse of palliative chemotherapy are not fully understood (11,12).
The provision of chemotherapy near the end of life is associated with a variety of factors, such as sociodemographic characteristics and clinical parameters (13,14). Wright et al. confirmed that in an intensive care unit (ICU), palliative chemotherapy was associated with increasingly aggressive treatment, which included the use of mechanical ventilation and cardiopulmonary resuscitation, and contributed to an increased death rate (15). The decision to stop palliative chemotherapy is among the top five practices that could reduce medical costs and improve patient care (16). In the previous study, we evaluated the use of and variables associated with chemotherapy in the last month and found that younger patients and those with lower performance status are more likely to receive palliative chemotherapy (17). However, we did not evaluate variables associated with the time from the last chemotherapy treatment to death in the last year. In the present study, we aimed to investigate the relationship between the time from the last chemotherapy treatment to death and the patient’s age, Charlson comorbidities, caregivers, and cancer type in the last year.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-273).
Methods
Data from patients who had died from metastatic or recurrent cancer between April 2007 and June 2019 at the Department of Integrated Therapy in Fudan University Shanghai Cancer Center in Shanghai, China, were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Research Ethics Committee of Fudan University Shanghai Cancer Center (NO.050432-4-1911: the registration number of ethics board). Informed consent was taken from all the patients. The inclusion criteria were as follows: patients whose cancer diagnosis information was available in the computerized medical record system at our hospital, patients who had received at least one cancer therapy, and patients who had received palliative chemotherapy as the final cancer therapy.
From each patient, the following data were collected: clinical information (cancer type, Charlson comorbidities, interval between the last chemotherapy treatment and death) and sociodemographic characteristics (family caregivers, gender, and age).
Data analysis
The interval between the last dose of palliative chemotherapy and death was analyzed using the Kaplan–Meier method. Differences in the time of the last PCT between different patient groups (based on age, Charlson comorbidities, caregivers, or cancer type) were examined using the log-rank test. Finally, Cox regression analysis was performed to further investigate the association between PCT and age, Charlson comorbidities, caregivers, and cancer type. The software package R version 3.1.3 was used to perform all the statistical analyses.
Results
Between April 2007 and June 2019, 605 patients died of advanced cancer at the Department of Integrated Therapy in Fudan University Shanghai Cancer Center. Among them, 250 (41.3%) received only the best supportive care during their final year of life, and 355 (58.7%) were treated with palliative chemotherapy in the last year (Table 1).
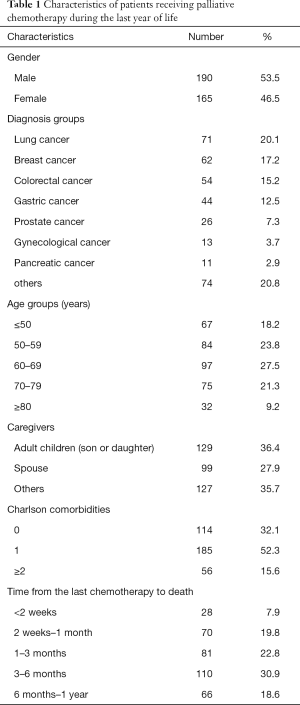
Full table
Cox regression analysis was performed to investigate the relationship between age, Charlson comorbidities, caregivers, cancer type, and the days from the final PCT to death.
The use of palliative chemotherapy varied significantly between the groups according to caregiver (Figure 1A, P<0.001). Patients whose caregivers were their adult children had the shortest interval between last time of chemotherapy and death and received palliative chemotherapy more frequently. Patients whose spouses were their caregivers received palliative chemotherapy less frequently.
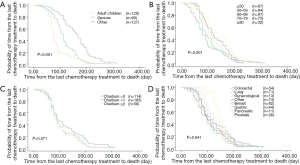
The use of palliative chemotherapy also varied significantly between age groups (Figure 1B, P<0.001). The differences were statistically significant for the group ≤50 years of age, without including other age groups. In the final year before death, 18% of patients ≤50 years of age were treated using palliative chemotherapy. They received this treatment more frequently and had the shortest interval between last treatment and death (P<0.001). The differences between the Charlson comorbidities groups and cancer type groups were not significant in relation to EOL palliative chemotherapy (Figure 1C,D).
Palliative chemotherapy was received by 98 patients (16.2%) during the final month of life. Patients aged 50 years old or younger underwent EOL chemotherapy most frequently (P<0.001). Among the patients, lung cancer was the most frequently diagnosed cancer type (18.4%), compared with other cancer types (P=0.632). Patients with comorbidity represented a higher proportion of patients who received palliative chemotherapy (P<0.05) (Table 2). Multivariate analysis using a logistic regression model revealed age ≤50 years (OR =2.26; 95% CI: 1.84–2.98) was an independent predictor of the administration of chemotherapy (Table 3).
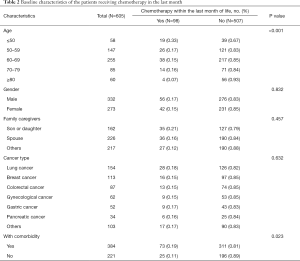
Full table

Full table
Discussion
Among the 605 identified patients in this study, 58.7% (N=355) received palliative chemotherapy during their last year of life, and 16.2% (N=98) were treated with chemotherapy during their final month of life. Patients aged 50 years or younger and those whose caregivers were their adult children showed an increased tendency to be treated with palliative chemotherapy in their final year of life and also had the shortest survival time after chemotherapy treatment. Palliative chemotherapy during the final year of life differed significantly according to the caregiver type and patient age. Treatment with palliative chemotherapy was also found to be independently associated with age (P<0.001) in the last month. The frequency of palliative chemotherapy in the final month before death was 15%, 13%, and 6% in the Netherlands, Germany, and Norway, respectively (1,9). Therefore, our figure of 16.2% (N=98) is similar to those of the previous studies.
Patients are treated with palliative chemotherapy for several reasons and we also found that EOL palliative chemotherapy hold more disadvantages. First, the physician’s ability to assess a patient’s prognosis has a major role in the final decision as to whether a patient will receive palliative chemotherapy. This is a difficult process that requires the doctor to involve the patient and caregivers in decision-making, including determining when the chemotherapy will start and when it will cease. Families and patients who discuss EOL decisions with their doctors received fewer aggressive medical interventions, including admission to an ICU department or palliative chemotherapy close to death, and the patients have a better quality of life in their final weeks (18,19). So, doctors, patients, and caregivers all play important roles in the decision process of choosing chemotherapy. However, in China, most patients and caregivers do not have sufficient opportunity to discuss the matter with their doctors because many doctors are busy with their daily clinical work and scientific research, and do not feel they have much time to spend in discussion with patients. Moreover, patients and their families sometimes need time to accept the disease prognosis, and they choose to struggle with cancer without a thorough discussion with the doctors.
Second, patients with a survival time >24 months were reported as more likely to be treated with palliative chemotherapy near the end of their lives (20). This might have occurred because the patients trusted in the effectiveness of the treatment and believed that a good-prognosis could be achieved, which might have resulted in unrealistic expectations. Such a situation could influence patients, families, and doctors to use costly and unnecessary drugs that might have potentially toxic side-effects, ultimately increasing anxiety and fatigue, and reducing quality of life for patients (21,22).
Third, there are currently many treatment options such as surgery, chemotherapy, or radiotherapy for cancer patients who are at an early stage of their illness; however, in patients with metastatic cancer, aggressive care, such as palliative chemotherapy near the end of life, might result from the lack of integrated multidisciplinary palliative care teams in hospitals, whose absence may leave the benefits and risks of EOL palliative chemotherapy unexamined. Multidisciplinary palliative care teams could promote thorough discussions between doctors, families, and patients, and consider patient information that could affect the judgment of a patient’s prognosis and the final decision for treatment, including age, performance score, tumor type, and tumor stage. In addition, multidisciplinary palliative care teams could help to judge whether a patient has a strong will to find a cure and if the patient is willing to receive palliative chemotherapy to prolong their life, despite the substantial side effects.
The present study showed that younger patients were treated more frequently, which might show that they were closer to death than older patients. The interval between the final dose of chemotherapy and the death of the patient varied significantly with age. When patients with cancer have a long survival time and have received first-, second-, third-, or fourth-line chemotherapy treatments, they might believe they are sensitive to chemotherapy, and may choose to receive palliative chemotherapy in the final year. Younger patients, in particular, usually have fewer comorbidities (Charlson <1) and a good performance status; therefore, they often choose to receive more types of cytotoxic drugs that might result in more difficulty for doctors, the patients, and their families when it comes to ceasing cancer treatments. However, older patients often have a poorer performance status and usually do not wish to be treated using palliative chemotherapy. This might be because older patients have more comorbidities (Charlson ≥2) and would thus be more susceptible to the adverse events of anticancer drugs that do not improve their survival (12). In the present study, the interval between the final dose of chemotherapy and death varied significantly by patient age, but not by Charlson comorbidities.
Family caregivers play a critical role in providing physical and emotional support to patients with advanced cancer (23). Caregivers can help to provide doctors with different perspectives through conversations about when to start or stop chemotherapy near the end of life. Filial piety is considered to be a very important Chinese cultural value that encourages children to try their best to prolong their parents’ lives, irrespective of the cost (24). Confucianism also encourages the decision-making process to be intentionally transferred from the individual to their family members (25). Thus, caregivers help to make final decisions instead of patients themselves, which contrasts with the situation in European countries, where patients usually make their own decisions. In consideration of filial piety, caregivers in China, especially children of the patient, possibly make the choice to receive palliative chemotherapy because they might equate treatment cessation with abandoning hope (26,27). Therefore, patients might endure the side effects of chemotherapy rather than abandoning hope of a cure. This idea is consistent with the results of our study, which showed that the interval between the final dose of chemotherapy and death varied significantly in the adult children caregiver group, in which patients were treated with palliative chemotherapy closer to death than in the spouse caregiver group.
Previous studies have found that factors such as tumor chemosensitivity, family economic status, and educational level are correlated with the use of chemotherapy during the end of life (28,29). Good palliative care guidelines and better communication between doctors, patients, and families could provide guidance when considering aggressive palliative chemotherapy.
The present study has a number of limitations. First, the retrospective nature of the study meant that it had to rely on the accuracy of the hospital records. Second, only data from patients who died in our hospital could be analyzed, meaning patients who died at other hospitals or at home were not included. Finally, other factors that might be associated with palliative chemotherapy, such as quality of life and chemosensitivity, were not considered.
Conclusions
The present study analyzed data from patients who died from metastatic cancer and were treated using palliative chemotherapy in their final year of life between April 2007 to June 2019 in Shanghai Fudan University. Of these patients, chemotherapy was provided to 58.7% of patients with advanced-stage cancer during their final year of life and was provided to 16.2% during their final month of life. The use of palliative chemotherapy in the final year of life varied significantly by patient age and caregiver type. In China, because of cultural factors, the final decision on when to commence palliative chemotherapy at the end of life lies with family members rather than the patients themselves. Chemotherapy does provide clinical benefit but might induce side effects; therefore, doctors, patients and caregivers need to cooperate to determine the best way to balance these considerations. Palliative care guidelines are required to aid decision-making, promote patient-physician communication, and personalize EOL decisions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-273
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-273
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-273). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Research Ethics Committee of Fudan University Shanghai Cancer Center (NO.050432-4-1911: the registration number of ethics board). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kao S, Shafiq J, Vardy J, et al. Use of chemotherapy at end of life in oncology patients. Ann Oncol 2009;20:1555-9. [Crossref] [PubMed]
- Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA 2016;315:272. [Crossref] [PubMed]
- Braga S. Why do our patients get chemotherapy until the end of life? Ann Oncol 2011;22:2345-8. [Crossref] [PubMed]
- Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 2008;26:3860-6. [Crossref] [PubMed]
- American Society of Clinical Oncology. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol 1996;14:671-9. [Crossref] [PubMed]
- Tang ST, Wu SC, Hung YN, et al. Trends in quality of end-of-life care for Taiwanese cancer patients, who died in 2000-2006. Ann Oncol 2009;20:343-8. [Crossref] [PubMed]
- Audrey S, Abel J, Blazeby JM, et al. What oncologists tell patients about survival benefits of palliative chemotherapy and implications for informed consent: qualitative study. BMJ 2008;337:a752. [Crossref] [PubMed]
- Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010;28:1203-08. [Crossref] [PubMed]
- Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778-84. [Crossref] [PubMed]
- Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315-21. [Crossref] [PubMed]
- Mohammed AA, Al-Zahrani AS, Ghanem HM, et al. End-of-life palliative chemotherapy: Where do we stand? J Egypt Natl Canc Inst 2015;27:35-9. [Crossref] [PubMed]
- Harrington SE, Smith TJ. The role of chemotherapy at the end of life: “When is enough, enough? JAMA 2008;299:2667-78. [Crossref] [PubMed]
- Wu CC, Hsu TW, Chang CM, et al. Palliative Chemotherapy Affects Aggressiveness of End-of-Life Care. Oncologist 2016;21:771-7. [Crossref] [PubMed]
- Zhang Z, Gu XL, Chen ML, et al. Use of Palliative Chemo- and Radiotherapy at the End of Life in Patients with Cancer: A Retrospective Cohort Study. Am J Hosp Palliat Care 2017;34:801-5. [Crossref] [PubMed]
- Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ 2014;348:g1219. [Crossref] [PubMed]
- Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 2012;30:1715-24. [Crossref] [PubMed]
- Zhang Z, Chen ML, Gu XL, et al. Palliative Chemotherapy Near the End of Life in Oncology Patients. Am J Hosp Palliat Care 2018;35:1215-20. [Crossref] [PubMed]
- Martoni AA, Tanneberger S, Mutri V. Cancer chemotherapy near the end of life: the time has come to set guidelines for its appropriate use. Tumori 2007;93:417-22. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Rochigneux P, Raoul JL, Beaussant Y, et al. Use of chemotherapy near the end of life: what factors matter? Ann Oncol 2017;28:809-17. [Crossref] [PubMed]
- Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195-8. [Crossref] [PubMed]
- Chen CH, Kuo SC, Tang ST. Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: systematic review and meta-regression analysis. Palliat Med 2017;31:406-18. [Crossref] [PubMed]
- Leighl NB, Butow PN, Tattersall MHN. Treatment decision aids in advanced cancer: when the goal is not the cure and the answer is not clear. J Clin Oncol 2004;22:1759-62. [Crossref] [PubMed]
- Cong Y. Ethical challenges in critical care medicine: a Chinese perspective. J Med Philos 1998;23:581-600. [Crossref] [PubMed]
- Htut Y, Shahrul K, Poi PJ. The views of older Malaysians on advanced directive and advanced care planning: a qualitative study. Asia Pac J Public Health 2007;19:58-67. [Crossref] [PubMed]
- Buiting HM, Rurup ML, Wijsbek H, et al. Understanding provision of chemotherapy to patients with end stage cancer: qualitative interview study. BMJ 2011;342:d1933. [PubMed]
- de Haes H, Koedoot N. Patient centered decision making in palliative cancer treatment: a world of paradoxes. Patient Educ Couns 2003;50:43-9. [Crossref] [PubMed]
- Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion charact eristics and care received near death: a prospective cohort study. J Clin Oncol 2012;30:4387-95. [Crossref] [PubMed]
- Rabow MW. Chemotherapy near the end of life. BMJ 2014;348:g1529. [Crossref] [PubMed]


