
Prediction model for death in patients with pulmonary tuberculosis accompanied by respiratory failure in ICU: retrospective study
Introduction
Tuberculosis (TB) remains a major public health problem in most of the developing world, and the incidence and mortality of TB remain high worldwide. TB is also a major health problem in China, which has the second-highest burden of TB in the world (1,2). Approximately 1 million new TB cases occurred in China every year (3), with an incidence and mortality of 459 and 9.8 per 100,000 populations respectively (4). Sputum smear microscopy and chest radiography were used to confirm the diagnosis of TB nowadays. however, this is being replaced by Xpert MTB/RIF, which allows a bacteriologically confirmed diagnosis of TB within two hours. But a clinical diagnosis of TB is often the norm. Bedaquiline added to anti-TB treatment as a new drug, however, the treatment success of severe TB remains low globally, at 55% (5). Severe TB patients had poor outcomes with no standard evaluation criteria, especially TB patients combined with respiratory failure. Respiratory failure is a common disorder in the intensive care unit (ICU) and it is associated with high mortality and morbidity (6). Although TB cases requiring ICU represent only 1–3% of all hospital admissions for TB, in-hospital TB mortality rates associated are higher (range: 29–83%) than for other infections (7,8). However, in China today, pulmonary TB is rarely considered as a primary cause of respiratory distress. Despite this, pulmonary TB with respiratory failure is associated with high mortality (9).
The prognosis in pulmonary TB patients accompanied by respiratory failure is believed to be poor. It is commonly believed that these patients have a poor outcome, and if they need mechanical ventilation would consume a large amount of intensive care resources, and more than 50% of these patients required mechanical ventilation (10). There has been persistently high mortality (69–80%) in patients with severe pulmonary TB and respiratory failure (11,12). Several studies have evaluated risk factors for death during TB treatment, the factors related to age, sex, and nutritional status of the host (13-15). However, few studies have attempted to identify the predictive factors associated with severe pulmonary TB in the ICU.
A better understanding of mortality trends and factors associated with mortality may lead to more informed suggestions about the prognosis of pulmonary TB patients with respiratory failure. Accordingly, we sought to evaluate the clinical features of pulmonary TB patients with respiratory failure and to identify the factors contributing to in-hospital mortality in these patients, and finally, to develop a simple model to determine prognostic factors for death.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-182).
Methods
Study population
Pulmonary TB patients were admitted to the medical ICU of Beijing Chest Hospital (Beijing, China), Chaoyang Fourth Hospital (Liaoning, China) and Hebi Third People’s Hospital (Henan, China) from May 2018 to May 2019, and all the patients with diagnoses of acute respiratory failure secondary to active pulmonary TB. The inclusion criteria were: (I) diagnosis of active TB (16,17); (II) accompanied respiratory failure (18); (III) admitted to the ICU ≥1 day; (IV) age more than 18 years. The exclusion criteria were: (I) non-tuberculous mycobacterial disease; (II) incomplete clinical data; (III) HIV infection (our center refers to HIV positive patients to a specialist hospital).
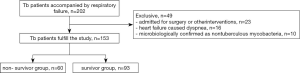
Of 202 patients diagnosed with active pulmonary TB who were admitted to the ICU during the study period, 49 were excluded: 23 were admitted for surgery or other interventions, 16 had heart failure caused dyspnea, 10 were microbiologically confirmed as nontuberculous mycobacteria (Figure 1). Of these acute respiratory failure secondary to active pulmonary TB patients, a total of 153 patients were divided into the survivor group and non- survivor group, and the factors related to patient death were collected.
Data collection
Standard clinical and laboratory parameters were collected and outcomes of survivor or non-survivor were recorded. Age, gender, body mass index, comorbid diagnoses, Acute Physiology, Age, and Chronic Health Evaluation (Apache) II score, and duration of symptoms when admission to ICU was recorded and compared between groups. In addition, radiological findings were documented, chest radiographs were reviewed on admission to ICU. Pathogenic examinations for M. TB were obtained from all patients in ICU were recorded. Anti-TB drug susceptibility testing was done using the concentration method. Blood cell count and serum biochemical analysis on laboratory investigations were recorded and compared. Arterial blood gas analysis at 0 and 24 hours after admission to ICU were collected. Furthermore, during hospitalization concomitant shock and organ failure were recorded.
Statistical analyses
Quantitative variables were expressed as median and range when not normally distributed, and as mean ± standard deviation (SD) when normally distributed. Categorical comparisons of death versus survival were performed using Pearson’s chi-square tests. To evaluate the risk factors for death during TB treatment, we compared clinical variables between the deceased and surviving groups using univariate comparison and subsequent multiple logistic regression. Multiple logistic regression was used to develop a prediction model with significant predictor variables from the bivariate analyses, and the model was assessed using receiver operator characteristic (ROC) analysis, with a P value of <0.05 as the criterion for statistical significance. All statistical analyses were performed using SPSS software (Version 26.0; SPSS, Chicago, IL).
Ethics
The study was approved by the ethics committee of Beijing Chest Hospital, Chaoyang Fourth Hospital, and Hebi Third People’s Hospital (No. 2018-ky-0102). The present study was conducted according to the principles of the Declaration of Helsinki.
Results
Demographic and clinical characteristics of the study participants
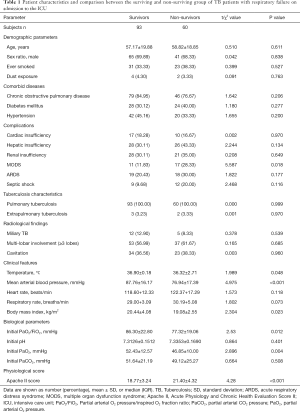
The baseline characteristics of both survivors and deceased patients are shown in Table 1. In total, 93 of 153 patients survived ICU admission (ICU mortality 39.22%) and 72 survived to hospital discharge (in-hospital mortality 52.94%). The median age of the 163 patients was 57.82±19.42 years (17.0–87.0 years), and there were 106 males (69.28%). Table 1 shows the risk factors for death due to pulmonary TB with respiratory failure following admission to the ICU. There were no differences between the survivors and fatalities in terms of age, sex, smoke history, dust exposure, and comorbid diseases. MODS in complications was significantly higher for non-survivors (17/60, 28.33%) than for survivors (11/93, 11.83%) (P=0.018; Table 1). But the cardiac, hepatic and renal insufficiency were not significantly different between the non-survivors and survivors groups (P>0.05). TB characteristics and radiological findings of Miliary TB (12/93, 12,90%), Multi-lobar involvement (≥3 lobes) (53/93, 56.99%) and Cavitation (34/93, 36.56%) were not significantly different in survivors group. The clinical features of temperature (36.32±2.71 vs. 36.90±0.18 °C, P=0.048), mean arterial blood pressure (MAP) (76.94±17.39 vs. 87.76±16.17 mmHg, P<0.001), Body mass index (19.08±2.55 vs. 20.44±4.08 kg/m2, P=0.023) of the non-survivors group was significantly lower than that of the survivors group. PaO2/FiO2, and PaO2 in patients admitted to the ICU were significantly lower for non-survivors (46.85±10.00 mmHg) than for survivors (52.43±12.57 mmHg) in univariate analyses (P=0.004). The Apache II score was significantly higher for non-survivors (25.12±4.16) than for survivors (22.06±5.34) in univariate analyses (P<0.001).
Full table
Mortality risk factors of TB patients with respiratory failure during ICU treatment
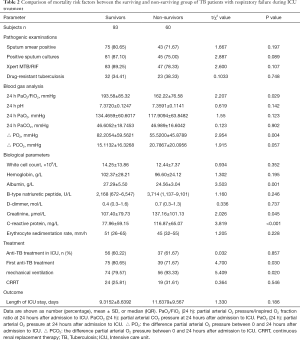
The mean length of the ICU stay for all patients were 10.21 days (range: 1–78 days). Table 2 shows that the PaO2/FiO2, the increased partial arterial O2 from 0 to 24 hours after admission to ICU at 24 hours were significant prognostic factors in univariate analyses. For all the patients, 77.12% were AFB-positive, 82.35% had positive sputum cultures and 84.97% had positive Xpert MTB/RIF test. The diagnosis of TB in the absence of direct microbiological proof was based on clinical and radiological evidence. All the patients had pathogenic examinations, about patients were sputum smear positive, there was no difference between deceased (71.67%) and surviving (80.65%) groups. There was no significant difference between two groups in positive sputum cultures, Xpert MTB/RIF and TB Drug-Resistant also. The albumin was significantly lower for non-survivors (24.56±3.04 g/L) than for survivors (27.29±5.50 g/L) in univariate analyses (P=0.001). Serum C-reactive protein (116.87±65.07 vs. 77.96±59.15 mg/L, P<0.001) and creatinine (137.16±101.13 vs. 107.40±79.73 µmol/L, P=0.045) in patients were significantly higher for non-survivors than for survivors. There were only about two-thirds of patients had anti-TB therapy in the ICU (surviving groups 60.22% vs. non-surviving groups 61.67%). there was a significant difference in new or retreatment between the two groups (80.65% first time-anti-TB treatment in survivor groups vs. 65.00% first time-anti-TB treatment in non-survivor groups). Non-survivors had significantly high ratios in mechanical ventilation (56/60, 93.33%, P=0.020). However, non-survivors had high ratios in CRRT than survivors, but there were no significant differences.
Full table
Multivariate logistic regression analyses for risk factors for death of patients with pulmonary TB with respiratory failure
In multivariate analyses, PaO2 (hazard ratio 0.928, 95% CI: 0.882–0.976, P=0.004), Albumin (hazard ratio 0.881, 95% CI: 0.792–0.980, P=0.019), Apache II score (hazard ratio 1.120, 95% CI: 1.017–1.234, P=0.022) and C-reactive protein (hazard ratio 1.012, 95% CI: 1.004–1.019, P=0.003) were independent risk factors for death of patients with pulmonary TB with respiratory failure during ICU treatment (Table 3).

Full table
Establishing a logistic model
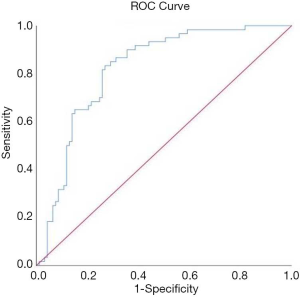
Of the aforementioned 14 most significant univariate variables MAP, albumin, Apache II score and C-reactive protein were selected as the input variables for the model. The model of the death risk grade of pulmonary TB with respiratory failure was Y=1.710−0.068*PaO2−0.163* albumin +0.215*APACH II+0.012* C-reactive protein. The value was Y=−0.494. If the Y value was greater than or equal to −0.494, the patients belonged to the deceased group, and if less than −0.494 the patients belonged to the survival group. Verified the prediction model by using the present clinical data. Receiver operator characteristics (ROC) regression modeling was done to retrospectively measure the overall predictive performance of the screening tool. The area under the ROC curve (AUR) is a measure of the accuracy, or the discriminative capacity of the screening test, and ROC curve analyses show the tradeoff between the differences in sensitivity and specificity of a test. A diagonal reference line (AUR =0.50) defines points where a test is no better than chance at identifying priority individuals (Figure 2). The sensitivity was 83.3%; specificity was 73.1%.
Discussion
This is a multicenter study to evaluate the death risk factors of TB patients with respiratory failure in China. The reported mortality rate in patients with pulmonary TB and respiratory failure is high. This study found a mortality rate of 39.21% in patients with TB accompanied by respiratory failure. This is within the 29% to 83% mortality rate reported for patients admitted to the ICU (19-21). Pathogenic examinations for M. TB were obtained from all patients, most patients had some degree of resistance to anti-TB drugs, there were about 2/3 fully susceptible. These results suggest that ICU patients’ resistance to anti-TB drugs were more serious and complex than in the common ward (21). In this retrospective study, factors contributing to mortality included the PaO2, albumin, Apache II score and C-reactive protein. We determined a simple scoring system that was predictive of fatality based on these independent factors.
As shown in this study, the PO2 of pulmonary TB patients with respiratory failure on admission to the ICU was lower for non-survivors than for survivors. As previous study reported, it is generally believed that the PaO2 level is associated with the severity of respiratory failure and prediction of outcomes (22). Belton et al. used hypoxia-specific tracer (18F) FMISO to investigate hypoxia, found that the presence of severe hypoxia within TB lesions in man, and IKK-β inhibition significantly decreased MMP-1 secretion in a dose-dependent manner in hypoxia (23), and hypoxia reduces inflammatory responses concerning macrophage motility and invasiveness, phagocytic capacity and most importantly bacterial killing (24). The lower the level of PaO2, the higher the death rate. Our data confirm evidence that low PaO2 is reliable in differentiating patients with poorer outcomes (12). The value of PaO2 level to screen for patients with active pulmonary TB with respiratory failure is that it enables early institutions of proven therapies.
Many previous studies reported that nutritional status is an important factor in TB treatment (25,26). Malnutrition leads to a decrease in immunity and M. TB is susceptible to infection; on the other hand, increased consumption of TB leads to malnutrition in the body. A previous cross-sectional study reported, 300 adult TB patients have surveyed China, they found that most male (90.8%) and female (58.4%) TB patients had insufficient daily protein intake. Li et al. found that more TB patients had albumin levels <35 g/L (27). Our results showed that pulmonary TB with respiratory failure patients albumin levels were even lower, ≤30 g/L. These results indicate that the results of this study are in line with the research conducted by Bhurayanontachai et al. (10), found that more TB patients command with respiratory failure had albumin levels <30 g/L. The body mass index and albumin was significantly lower in non-survivors than survivors, in multivariate analyses, albumin was an independent risk factor for death of patients with pulmonary TB with respiratory failure during ICU treatment, implying that the nutritional status was correlated with poor prognosis, thus demonstrating that albumin is good indicators of poor prognosis (10,28).
The Apache II score was given by the sum of the acute physiology score comprising 12 variables, age points and chronic health points. The patient’s condition was more serious, the prognosis was poorer, and the mortality rate was higher with higher scores. This score is widely used in the validation and prediction of clinical results of severe patients in the ICU, and there are some studies to indicate that the score can be used in TB patients (29). Other studies found a mean Apache II score of 21.2±6.5, indicating a mortality rate of 30–40% on ICU admission (10). In this study, the mean Apache II score was 21.40±4.32 in the fatalities group, indicating a mortality rate of 39.22% on ICU admission as previously reported (30). However, the high mortality rates in our study suggest that the Apache II score of pulmonary TB with respiratory failure on ICU admission and their mortality rate may be higher than for other diseases admitted to the ICU. The Apache II score was an independent risk factor for the death of pulmonary TB with respiratory failure during ICU treatment in multivariate analyses. However, another study found no correlation between mortality and Apache II score, and Apache II score made it less useful and potentially misleading for patients with PTB requiring intensive care in that study (30). As another previous study reported that acute respiratory failure caused by pulmonary TB necessitating mechanical ventilation has a high mortality rate and poor prognosis, particularly in patients with high Apache II score (31), our study suggests that the Apache II score may be used to predict their outcomes.
C-reactive protein is a highly sensitive biomarker of inflammation but is not specific to infection. Its serum levels increase in both infectious and non-infectious causes of inflammation (32). C-reactive protein has also been considered to be an important factor affecting patients’ outcomes. Our results showed that levels of C-reactive protein were significantly higher in non-survivors than survivors. The results of this study were comparable to the results of previous studies. Bajwa et al. found that high plasma C-reactive protein levels predict a favorable outcome in adults with ARDS, and they hypothesized that high C-reactive protein levels might reduce neutrophilic inflammation (33). Paradoxically, other reports showed that C-reactive protein levels are generally used as a biomarker for systemic inflammation, and higher values are associated with adverse outcomes (34). Albuquerque et al. found that C-reactive protein was strong discriminators of active TB and thus could be considered as biomarkers useful in discrimination different TB clinical forms (35). Another study found high C-reactive protein level was associated with sputum smear turn to negative (36). The association between C-reactive protein levels and outcome has not previously been investigated in TB patients with respiratory failure, so our study is the first to show that high C-reactive protein levels are associated with fatal outcome.
In our study, we determined a simple scoring system for pulmonary TB patients with respiratory failure isolated that was predictive of fatality based on these independent variables. This scoring system only applies to HIV negative patients. The sensitivity was 83.3%, and specificity was 73.1%. The prediction model will be beneficial for risk assessment in patients with TB and respiratory failure, enabling early institutions of proven therapies, allowing the prediction of outcomes, and facilitating clinical research. However, a larger sample is needed to verify this scoring system further.
Further study
One limitation of our study was its retrospective design. Firstly, limited ICU resources may have introduced a selection bias to the patients, and the number of patients may have been too small for analysis. Secondly, using past patient records was a limiting factor. As many risk factors are affecting the prognosis of pulmonary TB patients with respiratory failure, we still need prospective studies to explore the risk factors for mortality in this group of patients. This pilot study gave values of Apache II scores, C-reactive protein levels, PaO2, and albumin levels associated with surviving and non-surviving TB patients combined with respiratory failure that can then be used to design randomized clinical trials mainly focused on patients with a high risk of death.
Conclusions
Overall, TB patients with respiratory failure had a high mortality rate and poor prognosis, particularly those with high Acute Physiology and Chronic Health Evaluation II scores, high C-reactive protein levels and low PaO2 and low albumin. Our study suggests that paying attention to mortality-related risk factors would help reduce the mortality of patients. The use of these results to guide shared decisions between TB patients with respiratory failure and their clinicians may lead to improvements in the ICU and hospital outcome.
Acknowledgments
Funding: This work was supported by grants from Beijing Hospital Authority Youth Programme (grant no. QML20181603)
Footnote
Reporting Checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-182
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-182
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-182). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of Beijing Chest Hospital, Chaoyang Fourth Hospital, and Hebi Third People’s Hospital (No. 2018-ky-0102). The present study was conducted according to the principles of the Declaration of Helsinki.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu Y, Pang Y, Du J, et al. An Overview of Tuberculosis-Designated Hospitals in China, 2009-2015: A Longitudinal Analysis of National Survey Data. Biomed Res Int 2019;2019:9310917. [PubMed]
- Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet 2014;383:2057-64. [Crossref] [PubMed]
- World Health Organization. (2019) Global tuberculosis report 2019. World Health Organization, WHO/HTM/TB/2019.10.
- Wei X, Zou G, Walley J, et al. China tuberculosis policy at crucial crossroads: comparing the practice of different hospital and tuberculosis control collaboration models using survey data. PLoS One 2014;9:e90596. [Crossref] [PubMed]
- Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012;9:e1001300. [Crossref] [PubMed]
- Waller M, Murphy S, Krishnaraj N, et al. Respiratory failure and symptomatic hypercalcaemia complicating pulmonary tuberculosis. BMJ Case Rep 2009;2009:bcr. [Crossref]
- Kilaru SC, Prasad S, Kilaru H, et al. Active pulmonary tuberculosis presenting with acute respiratory failure. Respirol Case Rep 2019;7:e00460. [Crossref] [PubMed]
- Kim YJ, Pack KM, Jeong E, et al. Pulmonary tuberculosis with acute respiratory failure. Eur Respir J 2008;32:1625-1630. [Crossref] [PubMed]
- Nam H, Cho JH, Choi EY, et al. Current Status of Noninvasive Ventilation Use in Korean Intensive Care Units: A Prospective Multicenter Observational Study. Tuberc Respir Dis (Seoul) 2019;82:242-50. [Crossref] [PubMed]
- Bhurayanontachai R, Maneenil K. Factors influencing development and mortality of acute respiratory failure in hospitalized patient with active pulmonary tuberculosis: a 10-year retrospective review. J Thorac Dis 2016;8:1721-30. [Crossref] [PubMed]
- Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011;15:871-85. [Crossref] [PubMed]
- Filiz KA, Levent D, Emel E, et al. Characteristics of Active Tuberculosis Patients Requiring Intensive Care Monitoring and Factors Affecting Mortality. Tuberc Respir Dis (Seoul) 2016;79:158-64. [Crossref] [PubMed]
- Baussano I, Pivetta E, Vizzini L, et al. Predicting tuberculosis treatment outcome in a low-incidence area. Int J Tuberc Lung Dis 2008;12:1441-8. [PubMed]
- Duarte EC, Bierrenbach AL, Barbosa J Jr, et al. Factors associated with deaths among pulmonary tuberculo¬sis patients: a case-control study with secondary data. J Epidemiol Community Health 2009;63:233-8. [Crossref] [PubMed]
- Shimazaki T, Marte SD, Saludar NR, et al. Risk factors for death among hospitalised tuberculosis patients in poor urban areas in Manila, the Philippines. Int J Tu¬berc Lung Dis 2013;17:1420-6.
- Wang YT, Chee CB, Hsu LY, et al. Ministry of health clinical practice guidelines: prevention, diagnosis and Management of Tuberculosis. Singapore Med J 2016;57:118-24. [Crossref] [PubMed]
- World Health Organization (WHO). Definitions and reporting framework for tuberculosis. Geneva: WHO, 2013.
- Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117-25. [Crossref] [PubMed]
- Kim WY, Kim MH, Jo EJ, et al. Predicting Mortality in Patients with Tuberculous Destroyed Lung Receiving Mechanical Ventilation. Tuberc Respir Dis (Seoul) 2018;81:247-55. [Crossref] [PubMed]
- Erbes R, Oettel K, Raffenberg M, et al. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Respir J 2006;27:1223-8. [Crossref] [PubMed]
- Valade S, Raskine L, Aout M, et al. Tuberculosis in the intensive care unit: A retrospective descriptive cohort study with determination of a predictive fatality score. Can J Infect Dis Med Microbiol 2012;23:173-8. [Crossref] [PubMed]
- Wong JJ, Loh TF, Testoni D, et al. Epidemiology of pediatric acute respiratory distress syndrome in Singapore: risk factors and predictive respiratory indices for mortality. Front Pediatr 2014;2:78. [Crossref] [PubMed]
- Belton M, Brilha S, Manavaki R, et al. Hypoxia and tissue destruction in pulmonary TB. Thorax 2016;71:1145-53. [Crossref] [PubMed]
- Prosser G, Brandenburg J, Reiling N, et al. The bacillary and macrophage response to hypoxia in tuberculosis and the consequences for T cell antigen recognition. Microbes Infect 2017;19:177-92. [Crossref] [PubMed]
- Sari DK, Mega JY, Harahap J. Nutrition Status Related to Clinical Improvement in AFB-Positive Pulmonary Tuberculosis Patients in Primary Health Centres in Medan, Indonesia. Open Access Maced J Med Sci 2019;7:1621-7. [Crossref] [PubMed]
- Ren Z, Zhao F, Chen H, et al. Nutritional intakes and associated factors among tuberculosis patients: a cross-sectional study in China. BMC Infect Dis 2019;19:907. [Crossref] [PubMed]
- Li K, Yang C, Jiang Z, et al. Quantitative investigation of factors relevant to the T cell spot test for tuberculosis infection in active tuberculosis. BMC Infect Dis 2019;19:673. [Crossref] [PubMed]
- Hatsuda K, Takeuchi M, Ogata K, et al. The impact of nutritional state on the duration of sputum positivity of Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2015;19:1369-75. [Crossref] [PubMed]
- Chou CH, Lin GM, Ku CH, et al. Comparison of the APACHE II, GCS and MRC scores in predicting outcomes in patients with tuberculous meningitis. Int J Tuberc Lung Dis 2010;14:86-92. [PubMed]
- Loh WJ, Yu Y, Loo CM, et al. Factors associated with mortality among patients with active pulmonary tuberculosis requiring intensive care. Singapore Med J 2017;58:656-9. [Crossref] [PubMed]
- Ryu YJ, Koh WJ, Kang EH, et al. Prognostic factors in pulmonary tuberculosis requiring mechanical ventilation for acute respiratory failure. Respirology 2007;12:406-11. [Crossref] [PubMed]
- Tanrıverdi H, Tor MM, Kart L, et al. Prognostic value of serum procalcitonin and C-reactive protein levels in critically ill patients who developed ventilator-associated pneumonia. Ann Thorac Med 2015;10:137-42. [Crossref] [PubMed]
- Bajwa EK, Khan UA, Januzzi JL, et al. Plasma C-reactive protein levels are associated with improved outcome in ARDS. Chest 2009;136:471-80. [Crossref] [PubMed]
- Jaye DL, Waites KB. Clinical applications of C-reactiveprotein in pediatrics. Pediatr Infect Dis J 1997;16:735-46. [Crossref] [PubMed]
- Albuquerque VVS, Kumar NP, Fukutani KF, et al. Plasma levels of C-reactive protein, matrix metalloproteinase-7 and lipopolysaccharide-binding protein distinguish active pulmonary or extrapulmonary tuberculosis from uninfected controls in children. Cytokine 2019;123:154773. [Crossref] [PubMed]
- Komiya K, Goto A, Kan T, et al. A high C-reactive protein level and poor performance status are associated with delayed sputum conversion in elderly patients with pulmonary tuberculosis in Japan. Clin Respir J 2020;14:291-8. [Crossref] [PubMed]


