
Combined use of high-sensitivity ST2 and NT-proBNP for predicting major adverse cardiovascular events in coronary heart failure
Introduction
Coronary heart disease (CHD) is an inflammatory process mediated by many cytokines involved in innate and acquired immune activities (1). The participation of the interleukin (IL)-33/IL-1 receptor-like 1 (IL-33/ST2) axis in many cardiovascular diseases has been revealed in previous studies (2-8). These findings suggest that a novel mechanism of intramyocardial fibroblast-myocyte communication leads to ventricular remodeling and myocyte apoptosis. Moreover, an elevated serum soluble ST2 (sST2) level has been shown to be a significant marker of poor prognosis in both chronic heart failure (HF) (9,10) and acute myocardial infarction (11,12). A correlation has also been identified between plasma sST2 and many other cardiovascular biomarkers or hormones, including B-type natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), C-reactive protein (CRP), troponin T, creatine kinase, IL-6, noradrenaline, and aldosterone (13).
BNP is expressed by the myocardium in response to elevated atrial wall pressure and reduces venous return to the heart by influencing the vascular endothelium and the kidneys and suppressing reflex sympathetic activation (14,15). ProBNP is the active form of BNP in circulation (16). BNP and NT-proBNP have become important biomarkers in cardiovascular disease (17-20), including for acute coronary syndrome (ACS) and stable coronary artery disease (21-37).
In a study by the Northern New England Cardiovascular Disease Study Group, a full prediction model that included NT-proBNP and sST2 demonstrated significantly improved classification of in-hospital mortality in patients who received coronary artery bypass graft (CABG) in comparison with the Northern New England (NNE) model alone (38). Meanwhile, the Fragmin and fast Revascularization during InStability in Coronary artery disease (FRISC) II trial showed that NT-proBNP level could be used to identify patients who would benefit most from invasive treatment at an early point, particularly when used in combination with inflammatory factors such as IL-6 (39). High-sensitivity cardiac troponin T (hs-cTnT) and NT-proBNP were included in a multivariate model which showed higher discrimination ability in predicting mortality than the model without biomarkers (40). By combining hs-cTnT and NT-proBNP, the long-term (four-year) prediction of mortality risk in patients with stable CHD can be improved (41-43). NT-proBNP, hs-cTnT, and low-density lipoprotein cholesterol (LDL-C) are the three most significant biomarkers, and NT-proBNP and hs-cTnT possess superior value to other clinical biomarkers or variables (smoking, diabetes mellitus, and peripheral arterial disease) as predictors of cardiovascular death in patients with stable CHD.
Therefore, taking the roles of inflammatory and myocardial stretch in CHD into account, we hypothesized that a combination of sST2 and NT-proBNP might offer superior valuable in predicting the risk of major adverse cardiovascular events (MACEs) in CHD patients. To test this hypothesis, we performed an association analysis of sST2, NT-proBNP, and CHD risk in a case control study in patients from the Department of Cardiology of PLA General Hospital.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1046).
Methods
Study population
The study included patients with CHD confirmed by coronary angiography who were hospitalized in the Department of Cardiology of PLA General Hospital between January 2007 and January 2017. The requirement for informed consent was waived as the data of patients involved in this study were retrospectively analyzed. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the ethical review committee of the PLA General Hospital (S2016-070-02).
The inclusion criteria were as follows (3,5,8,9,12,17): (I) age ≥30 years; (II) experienced an episode of ischemic discomfort lasting a minimum of 30 minutes within 6 hours before admission; (III) exhibited at least 0.1-mV ST-segment elevation in 2 contiguous electrical cardiogram (ECG) leads; and (IV) willing and able to provide written informed consent.
The exclusion criteria were as follows (3,5,8,9,12,17): (I) evidence of cardiogenic shock; (II) serum creatinine (Cr) ≥1,300 µmol/L; (III) end-stage renal disease requiring dialysis; (IV) unable to follow the protocol or follow-up; (V) unable or unwilling to provide informed consent; (VI) a lack of samples for sST2 measurement; or (VII) any condition that might reduce the possibility of collecting the data needed to meet the study objectives. Blood samples were obtained from the patients by venipuncture and centrifuged before being stored at −80 °C. The same blood sample was used to determine NT-proBNP and sST2 levels.
Follow-up and outcome
Each patient was regularly followed up. When decompensation occurred, additional follow-up visits were made. In general, the patients were visited by nurses on a quarterly basis and by physician biannually. They also received elective follow ups from cardiologists, psychiatrists, and rehabilitation physicians. Patients who did not receive visits regularly were instead followed up via telephone. The follow-up procedure comprised a standard postal or telephone questionnaire to assess events such as death and acute myocardial infarction (AMI). When events were suspected to have occurred, the patients’ medical reports were obtained from treatment facilities or primary physicians and their case files were reviewed. MACEs in this study included major endpoint events, such as coronary revascularization, cardiovascular death, myocardial infarction, cerebral infarction/cerebral hemorrhage, non-cardiovascular death, peripheral arterial occlusion (extremities, kidney, and carotid artery), and HF.
sST2 assay
Measurements of sST2 were obtained from the stored serum samples with a high-sensitivity sandwich monoclonal immunoassay (Presage® ST2 assay, Critical Diagnostics, San Diego, CA, USA), which can accurately quantify sST2 levels, especially at low concentrations. For the Presage assay, the recombinant protein was used to generate antibodies based on the human cDNA clone for the complete sST2 sequence. The within-run coefficient of the sST2 assay was <2.5%, and the total coefficient of variation was 4%.
NT-proBNP assay
A standard electrochemiluminescence immunoassay (Elecsys proBNP, Roche Diagnostics, Indianapolis, IN) was used to measure serum NT-proBNP, as previously described (16). The range of the assay was 20 to 5,000 pg/mL, and it had intra- and inter-assay coefficients of variation of 2.9% and 6.1%, respectively. The measurement of NT-proBNP level was obtained from the same sample for sST2 level testing.
Statistical analysis
Categorical variables were compared using the χ2 or Fisher’s exact tests and expressed as percentages. Continuous variables were compared by Student’s t-test or the Mann-Whitney U test and expressed as the mean (standard deviation) or median (interquartile range) based on normal or non-normal distribution respectively. Associations between sST2 and NT-proBNP tertiles and relevant clinical variables were analyzed with analysis of variance (ANOVA) and Kruskal-Wallis test for symmetrical and asymmetrical continuous variables, respectively, and with χ2 test for categorical variables.
The association between MACEs and age, blood pressure, left ventricular ejection fraction (LVEF), body mass index (BMI), Cr, uric acid (UA), glucose (Glu), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), growth differentiation factor (GDF)-15, fibrinogen, Comorbidity, CHD type, multivessel disease, smoking, and treatment was analyzed using the Cox regression model. The assumption of linearity of the covariables sST2 and NT-proBNP was fulfilled using a quadratic term of sST2 and the logarithmic function of NT-proBNP in the Cox models.
The optimal cut-off points for sST2 and NT-proBNP were determined with ROC curvilinear coordinates and the tangent coordinates = sensitivity − (1-specificity), where the longitudinal axis was sensitivity and the transverse axis was 1-specificity.
The potential value of the inclusion of these biomarkers for predicting MACEs was assessed using three different forms of statistical analysis. In the bivariate regression analysis, the models were assessed for goodness of fit by applying the Hosmer-Lemeshow test, and the concordance index (C-statistic) was computed to compare the improvement in the discrimination ability of the sST2 and NT-proBNP biomarker model with that of the model without them. In binary regression analysis, the probability of MACEs of covariate was calculated based on statistically significant variables of risk factors for MACE from the COX regression analysis. In receiver operation curve (ROC) curve analysis, variables of risk factors, along with + sST2, + NT-proBNP, + ST2 and NT-proBNP serve as test variables, and MACEs as state variables to conduct the C-statistic.
Reclassification was mainly assessed using two statistics. First, net reclassification improvement (NRI) needed meaningful risk categories to be defined (tertiles of sST2 and BNP for the risk of MACEs were used). NRI was used to determine the incremental value of sST2, NT-proBNP, and sST2 + NT-proBNP including the variables for the prediction of outcomes at 3.9 years (42,43). NRI took into account changes in the estimated MACE prediction probabilities that implied a change between two categories. Second, integrated discrimination improvement (IDI) viewed the changes in the estimated MACE prediction probabilities as continuous variables. IDI = [(Pnew, events – Pold, events) – (Pnew, non-events – Pold, non-events)].
The fitness information of the model with the two biomarkers was tested by likelihood ratio test in the multiple logistic regression analysis, with MACEs as dependent variables, and the variables with statistically significant differences between MACEs and non-MACEs as covariates.
Statistical significance was indicated by two-sided P values of <0.05. SPSS (version 18.0 for Windows, SPSS, Inc., Chicago, IL, USA) statistical package was used to perform all statistical analyses.
Results
Baseline characteristics
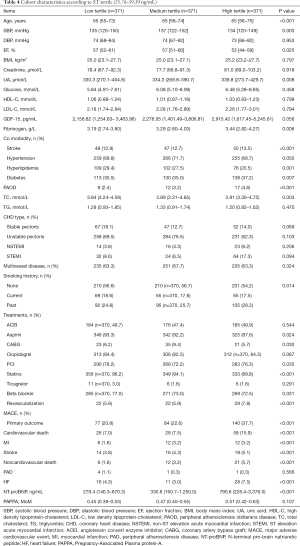
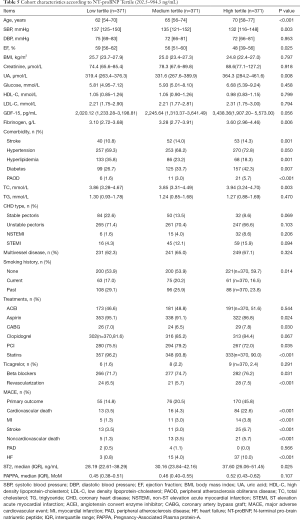
Data from 1,113 consecutive patients were analyzed in this study. The median age of the patients was 65 years (range, 32–95 years) old. The baseline characteristics of the included patients are shown in Table 1. In summary, the follow-up period lasted 3.9 years, during which time there were 113 cases of cardiovascular death, 30 cases of myocardial infarction, 49 cases of stroke, 39 cases of non-cardiovascular death, 6 cases of peripheral arterial occlusion, 55 cases of HF, and 73 cases of revascularization. A total of 365 cases had MACEs.
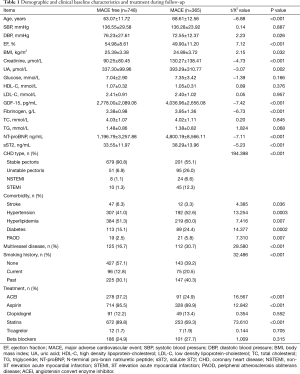
Full table
In Cox regression analysis, age, DBP, LVEF, BMI, Cr, UA, fibrinogen, NT-proBNP, and sST2 were found to be statistically significant continuous variables, while stroke, hypertension, hyperlipidemia, and diabetes were shown to be statistically significant categorical variables (P all <0.05). The treatment methods with statistical significance were aspirin, statins, beta blocker, revascularization, and PCI.
Cox regression and modelling
Cox regression analysis showed that age, NT-proBNP, systolic blood pressure (SBP), sST2, TC, CABG, EF, aspirin, UA, fibrinogen, and hypertension were significant independent predictors of MACEs. In the Cox regression analysis, NT-proBNP [hazard ratio (HR): 1.000024; 95% confidence interval (CI): 1.000011–1.000036; P<0.001] and sST2 (HR: 1.010; 95% CI: 1.001–1.018; P<0.05) were predictors of MACEs (Table 2).
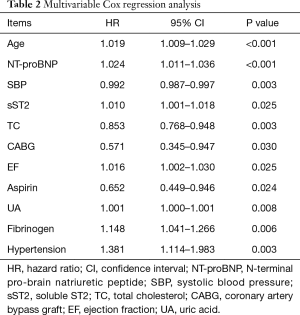
Full table
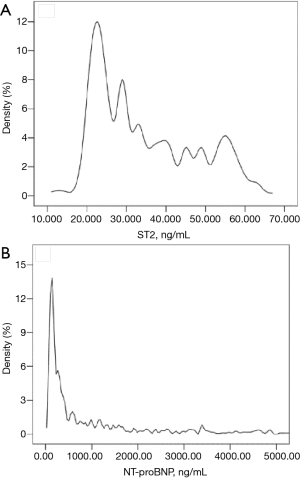
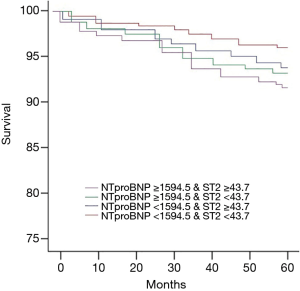
The best cut-off points for sST2 (43.7 ng/mL; 95% CI: 19.7–60.2; Figure 1A) and NT-proBNP (1,594.5 ng/mL; 95% CI: 85.4–17,864.4; Figure 1B) for predicting prognosis were identified by ROC curvilinear coordinates and the tangent coordinates. The patients were divided into four subgroups based on the aforementioned sST2 and NT-proBNP cut-off points, to establish to potential value of simultaneously assessing sST2 and NT-proBNP. The risk of MACEs in patients who had elevated levels of sST2 or NT-proBNP was higher than that in the reference group with low levels of each marker (Figure 2). Meanwhile, the risk for patients with elevated levels of both sST2 and NT-proBNP was markedly higher again. These results suggest that sST2 and NT-proBNP can more effectively identify patients who are at high risk of developing MACE when assessed in combination than when either one of these biomarkers is assessed alone.
Discrimination
The model with established MACEs risk factors (age, NT-proBNP, SBP, sST2, TC, CABG, EF, Aspirin, UA, fibrinogen, hypertension) had a C-statistic value of 0.742 (P<0.001). When separately incorporated into the model, NT-proBNP and sST2 both led to a significant improvement in the C-statistic for the prediction of MACEs of any cause (0.756 and 0.745 respectively). Furthermore, the model with the established MACEs risk factors that incorporated both biomarkers saw a significant increase in the C-statistic for predicting MACEs (Table 3).

Full table
Reclassification
MACEs patients were reclassified by risk category based on the occurrence of MACE during the follow-up period (Table 4). Following the separate inclusion of sST2, the model with established MACE risk factors and NT-proBNP, had an NRI of 1.03% (P<0.001), and an IDI = [(42.17−41.82) − (21.44−21.57)] of 0.48% (P<0.001). The NRI for patients who experienced MACEs was 0.66% (P<0.001), and the NRI for survivors was 0.37% (P<0.001) (Tables 4-7).
Full table
Full table

Full table

Full table
Calibration
The Hosmer-Lemeshow test was performed to determine the goodness of fit of the models. The results indicated that the models with and without the two biomarkers were well calibrated (P=0.355 vs. P=0.344).
Global model fit
Likelihood ratio tests were carried out to evaluate the global fit of the models. The model that incorporated both sST2 and NT-proBNP had a better global fit than the models with only the established MACEs risk factors and NT-proBNP (P<0.001).
Discussion
NT-proBNP has been well recognized as an important predictor in CHD. However, for patients with CHD, the use of NT-proBNP for risk assessment is still controversial. Recent studies have shown sST2 to be a useful biomarker for stratifying risk in various clinical contexts. sST2 signals through a complex involving IL-33 (44,45). The activity of sST2 in heart-related conditions has yet to be fully established; however, in one study, the disruption of the ST2 gene in an experimental murine model suggested that it may be associated with conditions such as cardiac hypertrophy and fibrosis (46).
sST2 is involved in the activation of T-helper type 2 (Th2) cells and the expression of Th2-associated cytokines. Previous studies have reported an association between higher levels of sST2 in plasma and increased risk of mortality and nonfatal adverse cardiac events. In 2003, Weinberg et al. measured the serum levels of sST2 in patients with chronic nonischemic HF upon admission and at 2 weeks after admission, and found that changes in sST2 level could predict poor prognosis of chronic heart failure (CHF) (3). Dieplinger et al. demonstrated that for patients with stable coronary artery disease (CAD), a higher level of sST2 could independently predict mortality of all causes over the long term and offer complementary prognostic value to hs-cTnT and NT-proBNP (44). Furthermore, in the Multi-Ethnic Study of Atherosclerosis (MESA), the investigators found that NT-proBNP surpassed other clinical risk factors as an independent predictor for incident CAD and cardiovascular disease (CVD). The Dallas Heart Study investigated a low-risk population, and it revealed that sST2 was associated with increased all-cause and cardiovascular mortality (46).
For patients with acute HF, sST2 is a powerful and reliable prognostic predictor (47). Meanwhile, for patients with CHD, it has also been shown that sST2 is an independent and complementary risk factor along with NT-proBNP (48). sST2 also served as an effective marker for the identification of CHD patients carrying risk of sudden cardiac death in a nested case-control study (49). In the current study, high-sensitivity sST2 provided independent prognostic information for predicting the occurrence of MACEs from all causes over the other variables studied before, including NT-proBNP. Thus, this study has unearthed new data regarding the value of sST2 in predicting the prognosis of CHD, as well as the complementary roles of both sST2 and NT-proBNP.
This method was evaluated by Ky in a younger, healthier group of subjects (50). The combined assessment of sST2 and NT-proBNP was discovered to moderately improve risk stratification within the group. Nevertheless, there was no significant improvement when sST2 was incorporated into a clinical model with NT-proBNP. Risk evaluation can be affected by differences in patient characteristics, such as age or disease severity. Furthermore, the length of follow-up in our study differed to those in previous analyses, which may offer another explanation for the differences observed between the studies. Meanwhile, the area under the curve (AUC) was 0.81 in Ky et al.’s study, compared with 0.742 in the current analysis (50).
This study analyzed the data from a group of CHD patients and incorporated sST2 (reflective of myocardial fibrosis and remodeling) and NT-proBNP (indicative of myocardial stretch) into a model with established risk factors of MACE, which led to an improvement in the risk stratification for death. When the model’s discrimination and reclassification abilities, calibration, and global fit were evaluated, it maintained a powerful ability to assess the risk of MACE occurrence among patients in the cohort.
Nevertheless, this study has some limitations. The levels of sST2 and NT-proBNP of the patients were measured based on frozen blood samples rather than fresh samples, which could have possibly influenced the absolute levels of the biomarkers. However, there is evidence to suggest that NT-proBNP and ST2 are not significantly affected by freeze-thaw cycles (51).
Although MACEs risk factors have been modified (52-54), at present there is only limited evidence to indicate that the risk of MACE can be reduced by lowering the levels of sST2 and NT-proBNP. Data for NT-proBNP from pilot studies and randomized clinical trials show that targeted therapy to reduce NT-proBNP levels may pave the way for the more effective use of proven CHD therapies, thus improving clinical outcomes (55,56). sST2 offers promise as a prognostic marker in the management of cardiovascular diseases. It can be used in combination with NT-proBNP to guide the treatment of cardiovascular diseases and help to reduce adverse clinical outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1046
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1046
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1046). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The requirement for informed consent was waived as the data of patients involved in this study were retrospectively analyzed. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the ethical review committee of the PLA General Hospital (S2016-070-02).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jabbar AY, Baydoun H, Janbain M, et al. Current concepts in the management of stable ischemic heart disease and acute coronary syndrome in patients with hemophilia. Ann Transl Med 2018;6:299. [Crossref] [PubMed]
- Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med 2008;205:339-46. [Crossref] [PubMed]
- Weinberg EO, Shimpo M, Hurwitz S, et al. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003;107:721-6. [Crossref] [PubMed]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol 2010;10:103-10. [Crossref] [PubMed]
- Dhillon OS, Narayan HK, Quinn PA, et al. Interleukin 33 and ST2 in non-ST-elevation myocardial infarction: Comparison with global registry of acute coronary events risk scoring and NT-proBNP. Am Heart J 2011;161:1163-70. [Crossref] [PubMed]
- Miller AM, Liew FY. The IL-33/ST2 pathway-A new therapeutic target in cardiovascular disease. Pharmacol Ther 2011;131:179-86. [Crossref] [PubMed]
- Milovanovic M, Volarevic V, Radosavljevic G, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res 2012;52:89-99. [Crossref] [PubMed]
- Eggers KM, Armstrong PW, Califf RM, et al. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J 2010;159:788-94. [Crossref] [PubMed]
- Felker GM, Fiuzat M, Thompson V, et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail 2013;6:1172-9. [Crossref] [PubMed]
- Parikh RH, Seliger SL, Christenson R, et al. Soluble ST2 for Prediction of Heart Failure and Cardiovascular Death in an Elderly, Community-Dwelling Population. J Am Heart Assoc 2016;5:e003188. [Crossref] [PubMed]
- Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 2004;109:2186-90. [Crossref] [PubMed]
- Wang YP, Wang JH, Wang XL, et al. Roles of ST2, IL-33 and BNP in predicting major adverse cardiovascular events in acute myocardial infarction after percutaneous coronary intervention. J Cell Mol Med 2017;21:2677-684. [Crossref] [PubMed]
- Aldous SJ, Richards AM, Troughton R, et al. ST2 has diagnostic and prognostic utility for all cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail 2012;18:304-10. [Crossref] [PubMed]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321-8. [Crossref] [PubMed]
- Parcha V, Arora P. Glycosylation of natriuretic peptides in obese heart failure: mechanistic insights. Ann Transl Med 2019;7:611. [Crossref] [PubMed]
- Semenov AG, Seferian KR. Biochemistry of the human B-type natriuretic peptide precursor and molecular aspects of its processing. Clin Chim Acta 2011;412:850-60. [Crossref] [PubMed]
- de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003;362:316-22. [Crossref] [PubMed]
- Morita E, Yasue H, Yoshimura M, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993;88:82-91. [Crossref] [PubMed]
- James SK, Lindback J, Tilly J, et al. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: a GUSTO-IV substudy. J Am Coll Cardiol 2006;48:1146-54. [Crossref] [PubMed]
- Sabatine MS, Morrow DA, de Lemos JA, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 2004;44:1988-95. [Crossref] [PubMed]
- James SK, Wallentin L, Armstrong PW, et al. N-terminal pro brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary disease-a GUSTO IV substudy. Circulation 2003;108:275-81. [Crossref] [PubMed]
- Windhausen F, Hirsch A, Sanders GT, et al. N-terminal pro-brain natriuretic peptide for additional risk stratification in patients with non-ST-elevation acute coronary syndrome and an elevated troponin T: an Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) substudy. Am Heart J 2007;153:485-92. [Crossref] [PubMed]
- Bonaca MP, Wiviott SD, Sabatine MS, et al. Hemodynamic significance of periprocedural myocardial injury assessed with N-terminal pro-B-type natriuretic peptide after percutaneous coronary intervention in patients with stable and unstable coronary artery disease (from the JUMBO-TIMI 26 trial). Am J Cardiol 2007;99:344-8. [Crossref] [PubMed]
- Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol 2003;41:1264-72. [Crossref] [PubMed]
- Wiersma JJ, van der Zee PM, van Straalen JP. NT-pro-BNP is associated with inducible myocardial ischemia in mildly symptomatic type 2 diabetic patients. Int J Cardiol 2010;145:295-6. [Crossref] [PubMed]
- Schnabel R, Rupprecht HJ, Lackner KJ, et al. AtheroGene Investigators. Analysis of N-terminal-pro-brain natriuretic peptide and C-reactive protein for risk stratification in stable and unstable coronary artery disease: results from the AtheroGene study. Eur Heart J 2005;26:241-9. [Crossref] [PubMed]
- Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007;297:169-76. [Crossref] [PubMed]
- Ndrepepa G, Braun S, Schulz S, et al. Sensitive troponin and N-terminal probrain natriuretic peptide in stable angina. Eur J Clin Invest 2011;41:1054-62. [Crossref] [PubMed]
- Mok Y, Sang Y, Ballew SH, et al. Premorbid levels of high-sensitivity cardiac troponin T and natriuretic peptide and prognosis after incident myocardial infarction. Am Heart J 2019;216:62-73. [Crossref] [PubMed]
- Morrow DA, Scirica BM, Sabatine MS, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol 2010;55:1189-96. [Crossref] [PubMed]
- Sadanandan S, Cannon CP, Chekuri K, et al. Association of elevated B-type natriuretic peptide levels with angiographic findings among patients with unstable angina and non-ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004;44:564-8. [Crossref] [PubMed]
- Wallentin L, Lindholm D, Siegbahn A, et al. Biomarkers in relation to the effects of ticagrelor in comparison with clopidogrel in non-ST-elevation acute coronary syndrome patients managed with or without in-hospital revascularization: a substudy from the Prospective Randomized Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2014;129:293-303. [Crossref] [PubMed]
- de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001;345:1014-21. [Crossref] [PubMed]
- Mega JL, Morrow DA, De Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol 2004;44:335-9. [Crossref] [PubMed]
- Koyama T, Munakata M, Akima T, Kanki H. Reduced Plasma NT-proBNP Levels Months after Myocardial Infarction Postconditioned with Lactate-Enriched Blood. Cardiology 2020;145:199-202. [Crossref] [PubMed]
- Tymińska A, Kapłon-Cieślicka A, Ozierański K, et al. Association of galectin-3 and soluble ST2 with in-hospital and 1-year outcomes in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Pol Arch Intern Med 2019;129:770-80. [PubMed]
- Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596-604. [Crossref] [PubMed]
- Polineni S, Parker DM, Alam SS, et al. Predictive Ability of Novel Cardiac Biomarkers ST2, Galectin-3, and NT-ProBNP Before Cardiac Surgery. J Am Heart Assoc 2018;7:e008371. [Crossref] [PubMed]
- Jernberg T, James S, Lindahl B, et al. NT-proBNP in unstable coronary artery disease -experiences from the FAST, GUSTO IV and FRISC II trials. Eur J Heart Fail 2004;6:319-25. [Crossref] [PubMed]
- Brophy JM, Dagenais GR, Boyer L, et al. Variability in High-Sensitivity Cardiac Troponin T Testing in Stable Patients With and Without Coronary Artery Disease. Can J Cardiol 2019;35:1505-12. [Crossref] [PubMed]
- Lindholm D, Lindbäck J, Armstrong PW, et al. Biomarker-based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol 2017;70:813-26. [Crossref] [PubMed]
- Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-72. [Crossref] [PubMed]
- Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med 2009;150:795-802. [Crossref] [PubMed]
- Dieplinger B, Egger M, Haltmayer M, et al. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem 2014;60:530-40. [Crossref] [PubMed]
- Chackerian AA, Oldham ER, Murphy EE, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol 2007;179:2551-5. [Crossref] [PubMed]
- Chen LQ, de Lemos JA, Das SR, et al. Soluble ST2 is associated with all-cause and cardiovascular mortality in a population-based cohort: the Dallas Heart Study. Clin Chem 2013;59:536-46. [Crossref] [PubMed]
- Socrates T, deFilippi C, Reichlin T, et al. Interleukin family member ST2 and mortality in acute dyspnoea. J Intern Med 2010;268:493-500. [Crossref] [PubMed]
- Januzzi JL Jr, Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol 2007;50:607-13. [Crossref] [PubMed]
- Pascual-Figal DA, Ordoñez-Llanos J, Tornel PL, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol 2009;54:2174-9. [Crossref] [PubMed]
- Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011;4:180-7. [Crossref] [PubMed]
- Ordonez-Llanos J, Collinson PO, Christenson RH. Amino-terminal pro-B-type natriuretic peptide: analytic considerations. Am J Cardiol 2008;101:9-15. [Crossref] [PubMed]
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. [Crossref] [PubMed]
- Damman K, Voors AA, Hillege HL, et al. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail 2010;12:974-82. [Crossref] [PubMed]
- Willenheimer R, van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite High-sensitivity ST2, NTproBNP, and prognosis in heart failure 37 sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation 2005;112:2426-35. [Crossref] [PubMed]
- Felker GM, Hasselblad V, Hernandez AF, et al. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J 2009;158:422-30. [Crossref] [PubMed]
- Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 2010;56:2090-100. [Crossref] [PubMed]


