Effects of dezocine, morphine and nalbuphine on electropain threshold, temperature pain threshold and cardiac function in rats with myocardial ischemia
Introduction
Myocardial ischemia (MI) is led by the unbalance between myocardial oxygen supply and myocardial oxygen requirements. Generally, angina is a common complication of MI. Increasing attention has been given to its clinical significance because sudden cardiac death would be caused by the disorder (1).
Several studies have been reported to illustrate difference of pain thresholds between on some pain tests in silent MI and symptomatic MI (2,3). However, few was to be reported about whether the different analgesic could have various influence to pain threshold. Analgesics are a large and diverse group of drugs that selectively inhibit and relieve various kinds of pain, which exact great importance on the postoperative recovery of patients and effect of treatment (4). They are potentially useful for one or more painful conditions by interacting with specific receptors of the central or peripheral nervous system (5). The main analgesics used in outpatient surgery or the treatment of moderate to severe pain are dezocine, morphine and nalbuphine. Anesthetic agents may differentially modulate pain perception (6). Particularly, it has been declared that morphine is a widely used opioid for treatment of moderate to severe pain (7).
Therefore, in the present study, the MI model was established by ligating the coronary arteries of rats. We applied the animal models that combine disease and syndrome, an important tool for pharmacodynamic evaluations, to investigated the effects of dezocine, morphine and nalbuphine on the electrical pain threshold (8), temperature pain threshold and cardiac function were investigated, providing a theoretical basis for the selection of safer and more effective analgesic drugs for patients with MI. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-19-460).
Methods
Experiment model
Experiments [(Healthy male Sprague-Dawley (SD) rats (SPF grade, 10–12 weeks old, and 250–300 g each)] were performed under a project license [license number: SCXK (Jun 2018-004)] granted by Tianjin Second Animal Experimental Center and was approved by institutional of Tianjin Second Animal Experimental Center [SCXK (Jun 2018-004)]. These rats were raised in a professional room with free drinking water and eating food. Room temperature was maintained at approximately 26 °C with a humidity of 40–70%, light was provided for 12 hours a day, and the room was well-ventilated.
Reagents and instruments
Main reagent
Ulatan (ethyl carbamate, analytical pure) was purchased from Sigma. The penicillin sodium injection was purchased from North China Pharmaceutical Co., Ltd. The dezocine injection (1 mL:5 mg) was purchased from Yangzijiang Pharmaceutical Co., Ltd. The sodium chloride injection (0.9%) was purchased from Hebei Siyao Group Co., Ltd. The morphine hydrochloride injection (0.5 mL:5 mg) and nalbuphine injection (1 mL:10 mg) were purchased from Hubei Yichang Renfu Pharmaceutical Group.
Main instrument
Electrocardiogram machine, PX9300, Futian, Japan; Neural stimulator, Stimuplex BL-410; Constant temperature water bath, Tianjin Huaxing Scientific Instrument Factory, TBS-5; Ultrasonic cardiograph, Siemens ACUSON Sequoia type 512 (8 mHZ probe); Refrigerator, Haier Co., Ltd.; Electronic balance, Mettler Toledo Instrument Co. Ltd.
Experimental methods
MI modeling
All rats were examined by electrocardiogram before the operation, and the T wave, S-T segment abnormal changes, or abnormal heart rhythms were discarded. Forty rats were randomly selected as the MI group. Then, 20% of urethane (dose: 5 mL/kg) was intraperitoneally injected for anesthesia. The thoracotomy was performed under sterile conditions, and the heart was exposed between the third and fourth ribs. After the operation, the electrocardiogram machine (rated voltage, 10 mm/mv; paper speed, 25 cm/s) was connected to record the changes of the electrocardiogram at different time points. The success of MI modeling was defined as achieving one of the following two conditions: (I) T wave towering and more than half of the R wave; (II) T wave towering and S-T segment displacement. Each rat was intramuscularly injected with 40,000 U/d of penicillin to prevent postoperative infection.
Grouping and administration
The purchased injections of dezocine, morphine and nalbuphine were prepared into a single dose of 0.5 mL of intravenous injection containing a 5-mg drug dose, and was intraperitoneally injected to these rats. Rats in the MI group were randomly divided into four subgroups, with 10 rats in each group: Mid group, a 2.5-mg/kg injection dose was given at 30 minutes after surgery; Mim group, an injection dose of 2.5 mg/kg of morphine was given at 30 minutes after surgery; Min group, an injection dose of 2.5 mg/kg of nalbuphine was given at 30 minutes after surgery; MI group, the same volume of 0.9% normal saline was injected at 30 minutes after surgery.
Measurement of pain threshold by electrical stimulation
Degreasing was carried out at 1.5–2.0 cm away from the rat tail tip, and the negative electrode of the nerve stimulator was connected to the degreasing site. Then, the positive electrode was connected and fixed at the root of the rat tail, and a 10% KCl solution was added to the junction to increase the electrical conductivity. Percutaneous electrical stimulation (2 Hz, 1 ms) was given to the nerve stimulator to observe the current magnitude, namely, the electrical stimulation tail rejection threshold (mA), which caused the tail rejection of these rats. The interval of the electrical stimulation was five minutes, and the values of five consecutive measurements were taken as the mean value of three measurements. The measurement was immediately conducted after administration, and at 1, 2, 3 and 4 hours, respectively. The percentage change in the tail rejection threshold. by electrical stimulation = (The tail rejection threshold by electrical stimulation after administration – the tail rejection threshold by electrical stimulation before administration)/the tail rejection threshold by electrical stimulation before administration ×100%.
Temperature pain threshold measurement
The water bath temperature was set at a constant temperature of 55 °C, immerse the area less than 1/3 of the end of the rat tail in the sink, and the time interval from immersion to the appearance of a spin was recorded, namely, the temperature stimulation spin threshold(s). The values of five consecutive measurements were taken as the mean values of three measurements. The percentage change in tail rejection threshold of temperature stimulation = (the tail rejection threshold of temperature stimulation after administration – the tail rejection threshold of temperature stimulation before administration)/the tail rejection threshold of temperature stimulation before administration ×100%. The temperature and pain thresholds were immediately measured at 1, 2, 3 and 4 hours after administration.
Cardiac function index measurement
For both the MI and control groups, at two hours after treatment, two-dimensional Doppler ultrasound cardiography was performed to record the left ventricular systolic and diastolic motion curve, and the left ventricular end-systolic diameter and end-diastolic diameter and respiration rate (RR) interphase, and calculate the heart rate (HR), ejection fraction (EF), cardiac output (CO) and isovolumetric diastolic volume (IRT). CO = Aortic orifice area × systolic aortic velocity × time × HR. Rats were anesthetized with 20% urethane, mean arterial pressure (MAP) was measured, and the hearts of these rats were extracted and weighed to calculate the relative heart weight.
Statistical analysis
All experimental data were expressed as “mean ± standard deviation” (
Results
Electrical stimulation rejection threshold comparison
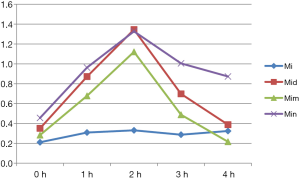
The electropain threshold values before and after administration are presented in Table 1. The response to electrical stimulation at each time point of MI (MI without treatment) was the lowest in the same time period, when compared to the other groups, and the peak was observed in this group at two hours after administration.

Full table
The change in the curve for the tail rejection threshold of electrical stimulation is presented in Figure 1. The higher the curve value was, the more the pain sense decreased (the threshold value increases). There was no significant difference in the MI group at each time point (P>0.05). Nalbuphine has the highest sensitivity, followed by dezocine and morphine. At one hour after administration, this gradually increased with morphine, but remained lower with dezocine and nalbuphine. At two hours after administration, all three drugs reached its peak. The difference between morphine and nalbuphine was not significant, while the decrease in pain with dezocine was the highest. After three hours of administration, the effect of nalbuphine decreased the slowest. That is the effect lasted for a long period of time. The effect of dezocine also decreased with time, while the effect of morphine basically diminished at four hours after administration.
Comparison of temperature-stimulated tail rejection thresholds
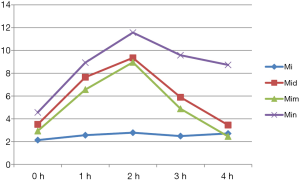
The temperature pain threshold value before and after administration is presented in Table 2. The temperature stimulation response corresponding to each time point of MI was the lowest at the same time period, when compared to the other groups, and the peak value appeared in all groups at two hours after administration. Figure 2 shows the variation curve for the tail rejection threshold under temperature stimulation. There was no significant difference between the MI group and the other groups at each time point (P>0.05). Nalbuphine had the highest sensitivity, followed by dezocine and morphine. At one hour after administration, the effect of morphine gradually increased, but this was still lower than that of dezocine and nalbuphine. There was no significant difference between dezocine and nalbuphine. At two hours after administration, all three drugs reached the peak value, and ranked in order from high to low, as follows: nalbuphine, dezocine and morphine. After three hours of administration, the effect of nalbuphine lasted for a long time and decreased the slowest. The effect of dezocine and morphine decreased with time, and the decrease trend of morphine was more significant.

Full table
Comparison of cardiac function indexes in rats
According to the above results for the electrical pain threshold and temperature pain threshold, the cardiac function indexes of each group of rats were measured at the peak of efficacy, that is, at two hours after administration. The HR, EF, CO, IRT and MAP results are presented in Table 3.

Full table
The HR comparison revealed that the Mid group had the fastest HR, while the Mim and Min groups had no significant difference, when compared to the MI group (P>0.05). EF was the lowest in the Mim and Mid groups. When compared with the MI group, there was no significant difference in CO between the Mim and Min groups (P>0.05), and the Mid group had the lowest output. IRT increased most significantly in the Min group, but no significant difference was found between the Mim group and MI group. For the MAP comparison, the Mid group presented with the most significant decrease, while the Mim and MI groups had no significant difference.
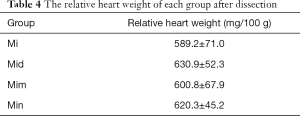
The hearts of rats in each group were dissected and weighed, and the results for the calculated the heart weight to body weight ratio are presented in Table 4. The weight gain in the Mid group significantly increased up to (630.9, 52.3) mg/100 g, but there was no significant difference between the Mim group and MI group.
Full table
Discussion
An animal model that combines disease and syndrome is an important method to explore the mechanism of disease occurrence and search for effective treatment methods. The advantages are as follows: (I) on the basis of the disease model, it has good reliability, stability and single factor; (II) it can introduce the time for model construction, and better reflect the law of disease development; (III) it is both macro and micro, and is practical and operational; (IV) it has more restrictions, and many uncertain factors clearer. The disadvantage is that there are great differences between animals and human beings in physiology and biochemistry, which needs to be verified by multiple parties before this can be applied in clinical practice. In the present study, the MI model was established by ligating the coronary artery. In the experiment, the measurement of cardiac function in the MI group confirmed that all indexes in the model group were significantly lower than those in the normal group. Some literatures have shown that severe MI can be induced as long as the infarct area is more than 20% (9,10). However, the disadvantage of this method is that the operation is difficult, and the postoperative mortality can reach more than 50%, which requires skilled operators (11).
Analgesics mainly act on the central or peripheral nervous system, selectively inhibits, and relieves all kinds of pain caused by tissue damage or potential damage, reduces the tension and anxiety caused by pain, and helps patients have a good rest, activity, and diet (12). Opioids have been commonly used as analgesics, but most of these are addictive, and have adverse reactions, such as respiratory depression (13). Therefore, in terms of drug selection, and in addition to its quick effects and good effects, these should also be safe.
Dezocine is a novel opioid receptor-antagonistic analgesic, which has been mainly applied as postoperative, visceral, and carcinogenic analgesics (14-16). In general, the peak value can reach within 10–90 minutes after intramuscular or intravenous injection, with an average terminal half-life of 2.4 hours (17,18). Its advantages mainly include the following: (I) The analgesic effect was similar to or slightly higher than that of morphine, but there were fewer mental dependence and related adverse reactions; (II) it has a capping effect on respiratory depression and so on; (III) it is a receptor agonist or receptor antagonist, and is less addictive (19). The common side effects include nausea, vomiting, sedation, giddiness, anorexia, disorientation, psychedelic, perspiration, and tachycardias (20). So coronary heart disease patients should take it with caution. The most common symptoms of MI were angina pectoris, presenting as paroxysmal and compressive pain in the front of the chest, accompanied by palpitations, shortness of breath, dyspnea, and other symptoms, which were mainly associated with strenuous exercise, mood swings and acute circulatory failure. In the present study, dezocine peaks in 2 hours, and the analgesic effect of electrical stimulation was better. However, for the rat HR, EF, CO, IRT and MAP, and relative heart weight was the most significant. Hence, analgesics should be chosen carefully for safety.
Morphine is a form of opioid drug morphine hydrochloride, a derivative of morphine, is a kind of anesthetic that can relieve acute pain in clinic, and it has a strong analgesic effect (21). It is also used for angina pectoris caused by myocardial infarction. Morphine mainly acts on the cerebral cortex, which can inhibit the pain area, respiratory center, and cough center. Morphine is usually injected subcutaneously and intramuscularly, and is absorbed rapidly. Furthermore, 60% of the total morphine can be absorbed within half an hour after injection, and rapidly metabolized to the lungs, liver, spleen, kidney, and other organs. Since morphine acts on opioid receptors in different brain regions, it has strong physiological dependence, and can easy cause addiction. Therefore, patients who need long-term medication should use this with caution. In the present study, morphine had the worst pharmacodynamic sensitivity and short duration, but its main effect was on the brain, and had the least impact on various cardiac function indexes in rats. However, due to its contraindication with use time, morphine has certain limitations.
Nalbuphine is short for nalbuphine hydrochloride injection, and is an opioid receptor agonist—antagonistic analgesic (22). It mainly acts on spinal cord kappa receptors, activates kappa receptors at the spinal cord level and kappa 3 receptors at the upper spinal cord, and is mainly used as relief to severe pain, such as burns, cancer, surgery, kidney, or biliary colic pain (23). It is also often used for myocardial infarction, angina pectoris, pain, etc. Its advantages are as follows: (I) a part of the antagonistic receptor has related side effects, which mainly reduce respiratory depression, nausea and vomiting, itching, and other adverse symptoms; (II) it does not easily increase the load to the heart, and does not increase blood pressure; (III) it has a quick response (5–10 minutes) and long action time (3–6 hours); (IV) it has a low incidence of adverse reactions and high safety, and can be used for postoperative analgesia, and gynecological and pediatric surgery analgesia (24). In the present study, the analgesic effect of nalbuphine was the earliest and longest, and it has the best analgesic effect in terms of temperature. In addition, in the cardiac function test of MI rats, nalbuphine has a small effect and high safety. Therefore, nalbuphine is a relatively good analgesic agent for patients with MI.
Conclusions
The present study provides a certain data basis for the selection of analgesics for patients with MI, but the specific application in clinical practice needs to be verified through multiple approaches, in order to ensure its safety and reliability.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-19-460
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-460
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-460). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license [license number: SCXK (Jun 2018-004)] approved by Tianjin Second Animal Experimental Center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang Z, Li C, Wang Y, et al. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J Mol Cell Cardiol 2018;125:185-94. [Crossref] [PubMed]
- Kurita A, Takase B, Uehata A, et al. Differences in plasma beta-endorphin and bradykinin levels be- tween patients with painless or with painful myocardial ischemia Am. Heart J 1992;123:304-9. [Crossref] [PubMed]
- Pedersen F, Pietersen A, Madsen JK. Elevated pain threshold in patients with effort-induced angina pectoris and asymptomatic myocardial ischemia during exercise test. Clin Cardiol 1989;12:639-42. [Crossref] [PubMed]
- Khan SP, Pickens TA, Berlau DJ. Perspectives on cannabis as a substitute for opioid analgesics. Pain Manag 2019;9:191-203. [Crossref] [PubMed]
- Piotrowska A, Kwiatkowski K, Rojewska E, et al. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia-evidence from in vivo and in vitro studies. Neuropharmacology 2016;108:207-19. [Crossref] [PubMed]
- Frölich MA, Zhang K, Ness TJ. Effect of sedation on pain perception. Anesthesiology 2013;118:611-21. [Crossref] [PubMed]
- Sverrisdóttir E, Lund TM, Olesen AE, et al. A review of morphine and morphine-6-glucuronide's pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci 2015;74:45-62. [Crossref] [PubMed]
- Yang M, Yao SJ, Zhang CJ. The detection of pain threshold and its influencing factors. Rehabilitation theory and practice in China 2009;15:603-5.
- Natsuaki M, Morimoto T, Yamaji K, et al. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc 2018;7:e008708. [Crossref] [PubMed]
- Choi SY, Kim MH, Cho YR, et al. Performance of PRECISE-DAPT Score for Predicting Bleeding Complication During Dual Antiplatelet Therapy. Circ Cardiovasc Interv 2018;11:e006837. [Crossref] [PubMed]
- Vadivelu N, Mitra S, Schermer E, et al. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth 2014;7:17-22. [PubMed]
- Zhang Q, You HJ. Advances in analgesia and its application in anesthesia. Chinese Journal of Pain Medicine 2016;22:241-4.
- Corder G, Castro DC, Bruchas MR, et al. Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci 2018;41:453-73. [Crossref] [PubMed]
- Feng C, Feng M, Jiao R, et al. Effect of Dezocine on IL-12 and IL-10 secretion and lymphocyte activation by culturing dendritic cells from human umbilical cord blood. Eur J Pharmacol 2017;796:110-4. [Crossref] [PubMed]
- Dong WS, Li J, Chen HJ, et al. Systematic evaluation of dizocine injection in the treatment of persistent pain in cancer patients. Chinese Journal of Pain Medicine 2016;22:123-7.
- Duan LX, Li XL. Pharmacological action and clinical application of dizocine juice injection. Chinese Journal of New Drugs 2004;13:851-2.
- Sun Q, Zhou W, Wu B, et al. Dezocine: a novel drug to prevent fentanyl-induced cough during general anesthesia induction. J Anesth 2012;26:470. [Crossref] [PubMed]
- Sun J, Hu W, Zheng Z, et al. Clinical Observation of Dezocine and Nalbuphine on Patient-controlled Intravenous Analgesia in Patients Undergoing Cesarean Section. China Pharmacyl 2018;29:1678-81.
- Picker MJ. Discriminative stimulus effects of the mixed-opioid agonist/antagonist dezocine; cross substitution by mu and delta opioid agonists. J Pharmacol Exp Ther 2007;283:1009-17. [PubMed]
- Li Xiuze, Xia Qing, Li Wei. Comparison of the Effects of Dezocine, Fentanyl, and Placebo on Emergence Agitation after Sevoflurane Anesthesia in Children. Int J Clin Pharmacol Ther 2015;53:241-6. [Crossref] [PubMed]
- Fan KC, Song LG. Evaluation of the effect of nabrephine hydrochloride on automatic intravenous analgesia after cesarean section. Clinical Research and Practice 2017;2:65-6.
- Torad FA, Hassan EA. Epidural Lidocaine, Nalbuphine, and Lidocaine-Nalbuphine Combination in Donkeys J Equine Vet Sci 2016;37:1-5.
- Imam MZ, Kuo A, Ghassabian S, et al. Intracerebroventricular administration of CYX-6, a potent µ-opioid receptor agonist, a δ- and κ-opioid receptor antagonist and a biased ligand at µ, δ & κ-opioid receptors, evokes antinociception with minimal constipation and respiratory depression in rats in contrast to morphine. Eur J Pharmacol 2020;871:172918. [Crossref] [PubMed]
- Deng M, Zhang JS, Gong H, et al. Clinical effect of nalbuthine hydrochloride injection on postoperative analgesia after cesarean section. Maternal and Child Health Care of China 2016;31:5519-21.