
Serum TSGF and miR-214 levels in patients with hepatocellular carcinoma and their predictive value for the curative effect of transcatheter arterial chemoembolization
Introduction
Primary hepatocellular carcinoma (PHC) is a common malignant liver tumor originating in hepatocytes or intrahepatic duct cells. The morbidity and mortality rates of PHC are among the highest of all malignant tumors (1,2). Early diagnosis and timely removal are the optimal approaches to treating PHC effectively, However, the majority of PHC patients are already at a later stage when they are diagnosed, meaning only a small portion are able to undergo routine removal surgery. For many PHC patients, transcatheter arterial chemoembolization (TACE) is the main option for conservatively prolonging survival time (3,4). Although TACE is the preferred treatment for patients with advanced PHC, there are still some patients who do not respond well and require repeated interventions (5). Therefore, there is a clinical need for a non-invasive and safe way by which to evaluate the effectiveness of TACE. Owing to its high sensitivity to tumor progression, tumor-specific growth factor (TSGF) is clinically used to evaluate the effect of tumor therapy (6). Current research has showed that microRNA is abnormally expressed in a variety of malignant tumors. As a member of the miRNA family, miR-214 is abnormally expressed in tumors originating from various tissues; especially in liver cancer cells, its levels are significantly reduced (7). This study aimed to investigate the changes in the levels of serum TSGF and miR-214 before or after TACE surgery, and to analyze their predictive value in the long-term efficacy of TACE, to provide a reference for clinical application.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1224).
Methods
General material
A retrospective analysis of the clinical data of 87 PHC patients were treated with TACE and from January 2013 to December 2015 in Xi'an Chang’an Hospital. According to the curative effect 1 month after TACE, PHC patients were divided into disease control group (n=56) and disease progression group (n=31). The clinical data of 87 PHC patients were treated with TACE and from January, 2013 and December, 2015 in Xi’an Chang’an Hospital and Hanzhong People’s Hospital were retrospectively analyzed. The inclusion criteria for patients were as follows: (I) PHC clinically and pathologically was diagnosed (8); (II) liver cancer resection could not be performed; (III) alpha-fetoprotein ≥400 µg/L; (IV) expected survival time ≥3 months; (V) first-time TACE operation; and (VI) there weren’t history of surgery, chemotherapy, or immune or targeted therapy. The following exclusion criteria were applied: (I) severe heart, liver, or kidney dysfunction; (II) another type of malignant tumor; (III) alpha-fetoprotein-negative liver cancer; (IV) severe coagulopathy; or (V) incomplete clinical data.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics committee of Chang’an Hospital of Xi’an and Ethics committee of Hanzhong City People’s Hospital board of (No. 20121226091H) and informed consent was taken from all the patients.
TACE treatment methods and evaluation criteria
The patients were placed in the supine position, and the right lower extremity artery was selected as the puncture point. Following local anesthesia, the artery was punctured using the Seldinger technique. Angiography was performed on the abdominal, hepatic, and superior mesenteric arteries. Then, the number of lesions, blood supply to the tumor, presence of vascular invasion, and arteriovenous fistula were observed and recorded. Next, 5F hepatic duct ultra-selective intubation was pushed to the hepatic artery, and an appropriate amount of chemotherapy drugs and super-liquid iodized oil mixed emulsion were injected. The blood supply at the lesion was confirmed by hepatic arteriography. After the blood supply at the focus is completely limited, the catheter was then removed and conventional wound dressing was performed. Postoperatively, the patients were also given liver protection and immunity enhancement.
During the treatment period, the effect was evaluated based on the imaging results (9), and the recheck interval was 4–8 weeks. The criteria for effect judgment were as follows: complete remission: the tumor has completely disappeared and no new lesions have appeared; partial remission: the reduction rate of the maximum diameter of the tumor exceeds 30%, and the maintenance time exceeds 4 weeks; stable: the reduction rate of the maximum diameter of the tumor is less than 30%, or the increase rate is less than 20%; and progression: the increase rate of the maximum diameter of the tumor exceeds 20% or new lesions have appeared. Disease control = complete remission + partial remission + stability.
Testing index
One day before TACE treatment or at one month after TACE treatment, 5 mL of peripheral blood was collected from each PHC patient into an anticoagulation tube. Then, the blood was centrifuged to remove the supernatant and divided into two parts: one part was stored at −20 °C for TSGF measurement, and the other was stored at −80 °C for the measurement of miR-214 expression.
- Determination of TSGF: enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of TSGF in the serum. The measurement was performed in triplicate, with the average value used as the final test result.
- Determination of miRNA-214: total RNA was extracted from the serum using an RNA extraction kit (Transgene, Beijing, China), according to the manufacturer’s instructions. Then, a UV spectrophotometer Jean Qi (Shanghai) Instrument Co., Ltd was used to check the OD value (A260/A280)=1.8–2.1, and the integrity of the RNA was checked with gel electrophoresis.
Reverse transcription (cDNA synthesis) was then performed for the high-quality RNA. Reverse transcription system (10 µL) (transgene, Beijing, China): 2× miRNA reaction mixture 5 µL, 0.1% BSA 1 µL, miRNA PrimeScript® RT enzyme mixture 1 µL, total RNA 0.5 µL, RNase ddH2O 2.5 µL. The reaction settings were: 37 °C 60 min, 85 °C 5 s, 4 °C 30 min.
Next, qRT-PCR (Quantitative Real-time PCR) was carried out to detect cDNA according to the manufacturer’s instructions. PCR system (10 µL): SYBR® Premix Ex Tap II (2×) 5 µL, upstream primer 0.4 µL, downstream primer 0.4 µL, ROX Reference Dye II (50×) 0.2 µL, cDNA 1 µL, ddH2O 3 µL. The PCR reaction settings were: polymerase activation at 50 °C for 5 min, pre-denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing and extension at 60 °C for 34 s, and the reaction was performed for 40 cycles. Dissolution curve drawing: 95 °C 15 s, 60 °C 60 s, 85 °C 15 s, 60 °C 15 s. β-actin was used as an internal reference gene. Three replicate wells were set for each sample, with the average Ct value taken for analysis. The relative expression levels were calculated using the 2−∆∆Ct method.
Follow-up
Patient follow-up in the form of telephone follow-up commenced on the day of pathological diagnosis. The outpatient reviews were conducted every three months for the first year after treatment, then once every six months for the next year, and at least once a year thereafter. The survival time and status of the patients at the time of follow-up (survival or death) were recorded.
Statistical analysis
SPSS 20.0 statistical software (IBM Corporation, USA) was used to perform data analysis. The measurement data were expressed as mean ± standard deviation, and the independent sample t-test was used to compare the two groups. The value of serum TSGF and miR-214 levels for predicting the efficacy of TACE were evaluated by using ROC curves and the area under the curve (AUC). Survival curves were generated using the Kaplan-Meier method, and the log-rank test was used to compare survival between the groups. A P value of <0.05 was considered to be statistically significant.
Results
Patient clinical data
According to the postoperative efficacy of TACE, the PHC patients were divided into the disease control group (n=56) and the disease progression group (n=31). The disease control group comprised 33 males and 23 females aged from 37–75 years, with an average age of 58.31±9.24 years. In terms of BCLC stage, there were 15 cases were stage A, 28 cases were stage B, and 13 cases were stage C. As for Child-Pugh stage, 49 cases were grade A and 7 cases were grade B. In the disease control group, 42 patients had a single lesion, and 14 cases had multiple lesions. The main tumor lesions measured 2.67–21.43 mm in diameter, with an average diameter of 8.94±2.61 mm. Meanwhile, the disease progression group comprised 22 males and 9 females aged from 45-81 years, with an average age of 65.47±8.68 years. In terms of BCLC stage, 8 cases were stage A, 15 cases were stage B, and 8 cases were stage C. As for Child-Pugh stage, 23 cases were grade A and 8 cases were grade B. In the disease progression group, 18 patients had a single lesion, and 13 cases had multiple lesions. The main tumor lesions measured 4.25–18.01 mm in diameter, with an average diameter of 11.56±3.29 mm.
Serum TSGF and miR-214 levels before or after TACE treatment
Before TACE treatment, the mRNA expressive of serum TSGF were increased in progression group, while the levels of miR-214 were decreased compared with control group (P<0.05). After TACE treatment, the mRNA expressive of serum TSGF were increased in progression group, while the levels of miR-214 were decreased compared with control group (P<0.05). Importantly, at one month after TACE treatment, the levels of serum TSGF were decreased in control group and progression group, while the expression of miR-214 was increased compared with in both groups before TACE treatment (P<0.05) (both P<0.05; Table 1).
ROC analysis of serum TSGF and miR-214 levels
In order to predict the efficacy of TACE, ROC analysis was performed in the disease control group to determine the value of serum TSGF and miR-214 levels. The AUCs for TSGF and miR-214 were 0.849 (95% CI: 0.759–0.938) and 0.807 (95% CI: 0.707–0.907), respectively, and the cutoff values were 181.32 U/mL and 0.63, respectively (Figure 1 and Table 2).
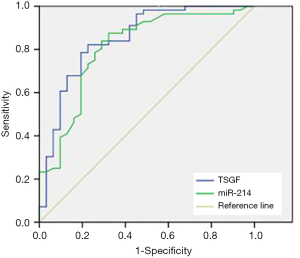
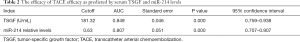
Full table
The relationship between serum TSGF and miR-214 levels and the three-year survival rate of patients after surgery
Of the 87 patients included in this study, 46 died within 3 years after treatment, and the 3-year survival rate was 47.13% (41/87). Based on the cutoff value, the PHC patients were divided into the high TSGF group (n=43) and the low TSGF group (n=44). The result of Kaplan-Meier curve analysis showed that the 3-year survival rate of the low TSGF group was 65.12% (31/44), which was up-regulated than that of the high TSGF group 30.23% (13/43) (χ2=5.014, P=0.025) (Figure 2, Table 3). Based on the cutoff value, the PHC patients were divided into the miR-214 high expression group (n=47) and the miR-214 low expression group (n=40).The 3-year survival rate of the miR-214 high expression group was 61.70% (29/47), which was up-regulated than that of the miR-214 low expression group 30.00% (12/40) (χ2=6.928, P=0.008) (Figure 2, Table 4).
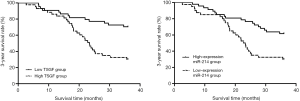

Full table
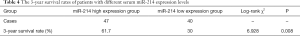
Full table
Discussion
TACE technology is constantly evolving, and its clinical use is conducive to the treatment of patients with advanced PHC. However, as single interventional therapies often cannot eliminate the lesion, the evaluation of the efficacy of interventional therapy is pivotal in guiding subsequent treatment (10). Currently, the main predominant methods for evaluating the clinical efficacy TACE after surgery are imaging and laboratory examination. However, there are certain flaws in imaging studies. Ultrasound examination, for instance, has a lower detection rate for recurrent of smaller lesions, whereas CT examinations can easily confuse iodized oil deposits and enhanced tumor lesions, which is not helpful for the detection of residual lesions. Despite MRI has a high detection rate, its application is limited because of the high costs it incurs (11). Alpha-fetoprotein detection is the most widely used laboratory test. However, the presence of alpha-fetoprotein-negative liver cancer makes its predictive value for the TACE postoperative efficacy controversial (12). Therefore, identifying routine blood tumor markers can be quickly and conveniently measured and predicting and evaluating the prognosis of patients is the focus of PHC research.
TSGF, which is a type of growth factor generated during the growth and spread of malignant tumors, helps to promote the formation and proliferation of capillaries in tumors and their surrounding tissues. It also releases the epithelial growth factor and stem cell growth factor into the blood; therefore, the levels of TSGF in serum hold an extremely high application value for tumor diagnosis and prognosis (13). In the research, after TACE treatment, the levels of serum TSGF were decreased in control group and progression group compared with in both groups before TACE treatment. The result of Kaplan-Meier analysis showed that the 3-year survival rate of the low TSGF group was up-regulated than that of the high TSGF group [65.12% (31/44) vs. 30.23% (13/43); χ2=5.014; P=0.025]. These results indicated that serum TSGF levels could possess a certain value for evaluating the efficacy of TACE. Xiong et al. (14) have found that the TSGF levels of PHC patients are significantly reduced after TACE treatment. Some earlier studies have found that miRNA is degraded by ribonuclease (RNase) present outside the cell. However, later studies have showed that miRNA widely and stably present in extracellular fluid. Since, the detection of miRNA expression in peripheral blood for the diagnosis and prevention of malignant tumors has been extensively studied (15). Studies have demonstrated that the expression of miR-214 play a differently in tumor tissues of different origins. For instance, its expression is significantly up-regulated in gastric cancer and pancreatic cancer, but downregulated in hepatocellular carcinoma (16). In the research, after TACE treatment, the expression of miR-214 was increased in control group and progression group compared with in both groups before TACE treatment (P<0.05). The 3-year survival rate of the miR-214 high expression group was up-regulated than that of the miR-214 low expression group [61.70% (29/47) vs. 30.00% (12/40); χ2=6.928; P=0.008]. These results suggested that the expression levels of serum miR-214 could hold a certain value in the evaluation of the curative effect of TACE. In contrast, Xiang et al. (17) have found that the expression levels of miR-214 in the peripheral blood of patients with gastric cancer are significantly increased, and that the patients with high expression of miR-214 have poor prognosis. This discrepancy might be related to the tissue-specific expression of miR-214.
In summary, after treatment with TACE, the levels of TSGF were decreased, while the levels of miR-214 were increased. Serum TSGF and miR-214 could be used as potential biomarkers for PHC diagnosis and prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1224
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1224
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1224). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics committee of Chang’an Hospital of Xi’an and Ethics committee of Hanzhong City People’s Hospital board of (No. 20121226091H) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget 2017;8:33911-21. [Crossref] [PubMed]
- Holzwanger DJ, Madoff DC. Role of interventional radiology in the management of hepatocellular carcinoma: current status. Chin Clin Oncol 2018;7:49. [Crossref] [PubMed]
- Bakopoulos A, Koliakos N, Tsilimigras DI, et al. Management of ruptured liver segment IV hepatocellular carcinoma: is transarterial embolization (TAE) superior to chemoembolization (TACE)?—the jury is still out. Ann Transl Med 2018;6:272. [Crossref] [PubMed]
- Wang Y, Shen X, Huang S, et al. Transcatheter arterial chemoembolization combined with elemene for the treatment of hepatic carcinoma. Transl Cancer Res 2018;7:1164-5. [Crossref]
- Yu X, Zheng H, Chan MT, et al. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med 2017;21:410-7. [Crossref] [PubMed]
- Abba M, Mudduluru G, Allgayer H. MicroRNAs in cancer: small molecules, big chances. Anticancer Agents Med Chem 2012;12:733-43. [Crossref] [PubMed]
- Anwar SL, Lehmann U. MicroRNAs: Emerging Novel Clinical Biomarkers for Hepatocellular Carcinomas. J Clin Med 2015;4:1631-50. [Crossref] [PubMed]
- Ge H, Zou D, Wang Y, et al. MicroRNA-377 Downregulates Bcl-xL and Increases Apoptosis in Hepatocellular Carcinoma Cells. Oncol Res 2017;25:29-34. [Crossref] [PubMed]
- Chen G, Lu L, Liu C, et al. MicroRNA-377 suppresses cell proliferation and invasion by inhibiting TIAM1 expression in hepatocellular carcinoma. PloS One 2015;10:e0117714. [Crossref] [PubMed]
- Zhang W, Che Q, Tan H, et al. Marine Streptomyces sp. derived antimycin analogues suppress HeLa cells via depletion HPV E6/E7 mediated by ROS-dependent ubiquitin-proteasome system. Sci Rep 2017;7:42180. [Crossref] [PubMed]
- Wang Y, Chen F, Zhao M, et al. The long noncoding RNA HULC promotes liver cancer by increasing the expression of the HMGA2 oncogene via sequestration of the microRNA-186. J Biol Chem 2017;292:15395-407. [Crossref] [PubMed]
- Xiong H, Li B, He J, et al. lncRNA HULC promotes the growth of hepatocellular carcinoma cells via stabilizing COX-2 protein. Biochem Biophys Res Commun 2017;490:693-9. [Crossref] [PubMed]
- Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene 2017;36:3528-40. [Crossref] [PubMed]
- Azizi M, Fard-Esfahani P, Mahmoodzadeh H, et al. MiR-377 reverses cancerous phenotypes of pancreatic cells via suppressing DNMT1 and demethylating tumor suppressor genes. Epigenomics 2017;9:1059-75. [Crossref] [PubMed]
- Wu H, Liu HY, Liu WJ, et al. miR-377-5p inhibits lung cancer cell proliferation, invasion, and cell cycle progression by targeting AKT1 signaling. J Cell Biochem 2018. [Epub ahead of print]. [PubMed]
- Xiang ZL, Zeng ZC, Fan J, et al. The expression of HIF-1alpha in primary hepatocellular carcinoma and its correlation with radiotherapy response and clinical outcome. Mol Biol Rep 2012;39:2021-9. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)



