
The effect of Helicobacter pylori infection on the decline of lung function in a health screening population
Introduction
Helicobacter pylori (H. pylori) is the leading cause of several gastric diseases, including superficial gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma. Since reports indicated that H. pylori infection was associated with coronary heart disease (1), subsequent epidemiological studies also associated H. pylori infection with extra-gastric diseases, such as cardiovascular, neurologic, hematologic, allergic, metabolic, and other diseases (2-4).
However, the relationship between chronic obstructive pulmonary disease (COPD) and H. pylori infection remains controversial. A previous study demonstrated that patients with chronic bronchitis tested significantly higher for H. pylori seropositivity than controls (83.3% vs. 60%; P=0.007) (5). Additionally, the rate of H. pylori seropositivity was reported to be higher in patients with COPD than in controls (54.7% of 58 patients with COPD, 23.5% of 17 controls (P=0.026) (6).
Recently, large epidemiological studies have shown an association between H. pylori seropositivity and COPD. Specifically, previous findings revealed that H. pylori IgG levels correlated with the severity of COPD (7). Conversely, a population-based cross-sectional study reported no association between H. pylori exposure and COPD, measures of allergic disease, or decline in lung function (8). These results support our previous study of a population with high H. pylori infection rates that demonstrated no relationship between COPD and H. pylori seropositivity (9). However, a meta-analysis that consisted of 16 studies suggested that H. pylori infection was associated with an increased risk of COPD [odds ratio (OR) 2.07, 95% CI: 1.81–2.36, P=0.05] (10).
Since H. pylori infection rates vary by geographic area, age, and ethnicity, we conducted a large-scale longitudinal data analysis in Korea where H. pylori infection is prevalent. Herein we investigated the relationship between H. pylori infection and the rate of lung function decline, including the risk for COPD. In addition, we evaluated the impacts of medical eradication of H. pylori on lung function decline and the risk of COPD to further clarify the relationships.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-850).
Methods
Patients and data
We conducted this retrospective cohort study using the Gene-Environment of Interaction and phenotype (GENIE) data. The GENIE cohort consisted of participants who had visited Seoul National University Gangnam Center in South Korea for health checkups, agreed to participate in genetic studies, and donated blood samples (11).
Data were obtained from 2004 to 2015 in adults at least 40 years old, and the clinical information was collected including age, sex, height, weight, comorbidities, smoking status, spirometric and laboratory data, and esophagogastroduodenoscopy (EGD) results. Spirometry and H. pylori-specific IgG concentrations were measured annually for up to 12 years. Smoking-related variables were obtained by questionnaire. Patients who had undergone at least one H. pylori IgG test and who had spirometry for more than two years were included in the study. We excluded patients who were diagnosed with lung cancer during the follow-up period or who underwent pulmonary resection. Spirometry was performed according to the standard guidelines of the American thoracic society (12).
H. pylori-specific IgG concentrations were measured using two different tests depending on the study year. A commercially available enzyme-linked immunosorbent assay (RADIM, Pomezia-Rome, Italy) was used according to the manufacturer’s protocol prior to April 2013. We included only positive (≥30 U/mL) or negative (<15 U/mL) results in this analysis excluding equivocal results (15–30 U/mL). H. pylori-specific IgG concentrations were measured with HPG kits (Immulite 2000 Chemiluminescent Microparticle Immuno Assay SIEMENS, UK) from April 2013 until the end of the study. H. pylori IgG levels higher than 1.10 IU/mL were regarded as positive, and values that ranged from 0 to 0.9 IU/ mL were considered negative in the Immulite 2000 assay. We defined a recently infected person as a subject whose H. pylori-specific IgG value changed from negative to positive during the follow-up period.
Outcome measures
Using the baseline H. pylori-specific IgG concentrations, we determined whether H. pylori seropositivity was associated with the incidence or development of COPD. COPD was defined as a prebronchodilator FEV1/forced vital capacity (FVC) ratio of <0.7. We also compared the annual FEV1 or FVC reduction rates of seropositive and seronegative subjects.
Moreover, the difference in the rate of decline in pulmonary function of seropositive patients was evaluated by stratifying the patients into two groups: (I) the H. pylori-treated group and (II) the non-treated group. The treated group included the patients who completed the course of eradication regimen drugs such as a proton pump inhibitor (PPI), clarithromycin, and amoxicillin.
A subject whose H. pylori-specific Ig G value changed from negative to positive during the follow-up period was defined as a recently infected person.
Statistical analysis
Data are presented as the mean ± standard deviations (SDs) for continuous, normally distributed variables or medians with interquartile ranges (IQRs) for non-normally distributed variables. Categorical variables were analyzed using the chi-square test, and a t-test was used to compare the differences between normally distributed, continuous data. P<0.05 (two-tailed) was considered statistically significant. A random intercept and random slope model were used to estimate the annual lung function decline. The data were adjusted for multiple covariates, including age, sex, height, and smoking pack-year. We performed statistical analyses using STATA software version 15.0 (StataCorp, College Station, TX, USA).
Ethical statement
The present study was approved by the Institutional review board (IRB No. H-1804-028-934) and was in accordance with the 1964 and later modifications of the Helsinki declaration (as revised in 2013) on the use of human subjects for research. Written informed consent was obtained from the patient before GENIE cohort registration.
Results
A total of 3,843 patients were followed-up for 2–12 years (median follow-up, 7 years; IQR, 5–9). Among these participants, 224 subjects (5.83%) were excluded due to equivocal H. pylori IgG tests. Therefore, we included 3,619 subjects in our analysis. The baseline characteristics of the study subjects are presented in Table 1. The mean age of patients was 48.57 years; 2,226 (61.5%) were men, and 602 (16.7%) were current smokers. The mean FEV1 was 3.13±0.66 L (104.2% of the predicted value), and the mean FVC was 3.86±0.82 L (96.66% of the predicted value). Further, 1,849 (51.1%) patients were seropositive, and 1,770 (48.9%) were seronegative according to the first serology test.
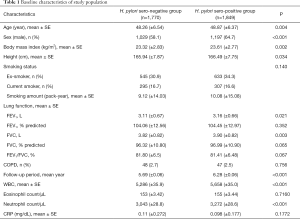
Full table
H. pylori infection and prevalence of COPD
The initial spirometry revealed that a total of 95 (2.6%) patients had COPD; however, the prevalence of COPD did not differ according to the seropositivity for H. pylori (2.5% in seropositive group vs. 2.7% in seronegative group; P=0.756). Although 76 patients (2.1%) were newly diagnosed with COPD during the total observation period, there were no significant differences in the development of COPD between the seropositive group (37 patients, 2.0%) and the seronegative group (39 patients, 2.2%) (P=0.728).
The impact of H. pylori infection on the rate of decline in lung function
When comparing the annual decline rates of the mean FVC and FEV1 according to the seropositivity of H. pylori, we found no significant difference in the annual decline rate of the mean FVC between the two groups (35.38±0.90 mL/year for seronegative group vs. 34.34±0.81 mL/year for seropositive group, P=0.389) (Table 2, Figure 1) or in the annual decline rate of the mean FEV1 (39.23±0.75 mL/year in seronegative group vs. 37.49±0.68 mL/year in the seropositive group, P=0.086). To minimize the limitation of H. pylori-specific IgG in identifying recent infection, we also performed subgroup analyses in 143 patients with recent seroconversion to seropositive status during the observation period. Nevertheless, there were no differences in the mean FVC (40.05±2.54 mL/year) or FEV1 (34.62±3.13 mL/year) rates of decline (Table 3).

Full table
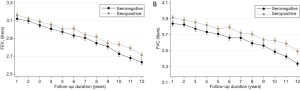

Full table
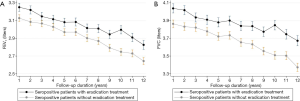
The impact of eradication on the decline of lung function
A total of 460 of the 1,849 seropositive patients (24.9%) received antibiotics and PPI for H. pylori eradication therapy. The annual mean FVC decline rate (34.51±1.41 mL/year) did not significantly differ from that of subjects without eradication treatment (34.26±0.99 mL/per year, P=0.603) (Table 4). Also, there was no significant difference in the annual decline rate of FEV1 between the two groups (37.29±1.15 mL/year for H. pylori treated group vs. 37.37±0.81 mL/year for the group without H. pylori treatment (P=0.954) (Figure 2).

Full table
Discussion
This large-scaled longitudinal analysis demonstrated that H. pylori infection did not affect the annual decline rates of FEV1 or FVC, and infection was not associated with the incidence of COPD. Although several reports indicate that H. pylori infection increases the risk of COPD, the present study, in conjunction with our previous study, indicated that H. pylori infection was not associated with COPD (9). Additionally, the eradication of H. pylori did not affect the rate of decreased in lung function and the development of COPD.
This study presents some strengths compared with previous reports on the association between H. pylori infection and COPD. First, this was a large-scale population cohort study with a long follow-up period in a high prevalence area of H. pylori infection. Secondly, the study population, GINIE cohort, consisted of a health screening population and followed an organized system that resulted in a low dropout rate. Numerous publications have already demonstrated the data quality of the study population. This suggests that the longitudinal data in this study may contain fewer biases such as the high drop-out rates seen in retrospective cohort studies. Furthermore, the effects of the eradication treatment of H. pylori infection were examined on main outcomes. To date, no studies have confirmed whether the rate of lung function is affected with H. pylori eradication.
As the prevalence of H. pylori infection varies by region, the effects of H. pylori infection on extra-gastric diseases are also expected to be variable. For example, the mean prevalence of H. pylori infection in the USA was 35% (range, 22–48%) between 1990 and 2006 with no increasing or decreasing infection rates over this time period (13). In this study, the prevalence of H. pylori infection was 51.1%, similar to previous studies conducted in Korea that reported a prevalence of H. pylori infection from 50 to 60% (14,15).
As for the physical characteristics of study population compared with previous reports (16-18), even though H. pylori seropositive group showed statistically higher values in age, BMI, and height, the absolute difference in each variable seems to be too small to suggest clinical significance in general population and some studies reported that the effect of H. pylori infection on height disappeared after a multivariate analysis (16,17,19,20). Male predominance in the H. pylori seropositive group appeared in this study, similar to several large sample studies that have reported that men were more positive on serum or biopsies than women (21,22).
Currently, Sze et al. reported the most extensive study including patients with COPD in a lung health study (LHS) on the relationship between H. pylori seropositivity and COPD (7). With the observation over 11 years, they showed that the absolute FEV1 value in seropositive patients was lower than that in seronegative patients even though the relationship disappeared when the FEV1 (% predicted) value was used. The authors asserted that H. pylori infection was associated with reduced lung function related to the effects of the bacterium on lung growth earlier in life and systemic inflammation. Contrary to the report, our study did not show any significant difference in the rate of lung function (both absolute value and % predicted value) in the seropositive population. Furthermore, although not statistically significant, a slower rate of decline in FEV1 was observed in the seropositive group (P=0.08).
However, we should consider some different aspects in both studies. First, our study enrolled a healthier general population. The baseline lung function was higher in this population and only 2.6% of total subjects had COPD at the enrollment (mean FEV1 2.54 L, 83.87% predicted), while the analysis report of LHS targeted mild to moderate COPD patients (mean FEV1 2.80 L, 78% predicted) (7). Therefore, even the impacts of H. pylori infection may present, the influence on the total lung function may be limited or negligible in a healthier population than in established COPD. Another point is the difference in seropositivity in the study population. As shown in this study, the seropositivity of H. pylori was so prevalent that more than half of the population had a current or past infection of H. pylori, a rate much higher than the 18% reported in the LHS study.
The induction of systemic inflammation by H. pylori was proposed as the mechanism underlying the association between COPD and H. pylori infections. The infection can trigger a low-grade inflammatory state, including the type 1 helper immune response and suppression of the type 2 T helper cell immune response (23). This polarized T-helper cell response has explained the relatively low prevalence of asthma and the high prevalence of COPD in this seropositive population. In addition, recently, studies are focusing on the effects of H. pylori on the intestinal microbiome (24). However, in a high prevalence area, the inflammation related to H. pylori may present such a weak or common signal that it may not be a major contributor to reduce lung function.
In this study, for the first time, we evaluated the effects of eradication of H. pylori on changes in lung function. However, the effect was not significant. This may suggest that the other factors including subsequent inflammation, are more critical rather than the initial H. pylori infection itself. However, as the main results were negative and the detailed inflammatory markers except CRP were not available in this study, further analysis and interpretation could not be performed.
Despite the interesting findings of this study, some limitations should also be considered. First, we considered the limitation of seropositivity in detecting current infection of H. pylori and the discrepancy with results using endoscopic biopsy samples (9,25). Unfortunately, endoscopic biopsy results of H. pylori were not available in the current study. Second, the study population may be relatively young to analyze more COPD patients considering that the prevalence of COPD increases with age (26) and other association studies (10,27).
Conclusions
In the present study using health screening general population, seropositivity defined infection or the eradication treatment of H. pylori were not associated with the decline of lung function or progression to COPD. H. pylori infection may not be a significant factor contributing to deteriorating lung function.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-850
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-850
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-850). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Institutional review board (IRB No. H-1804-028-934) and was in accordance with the 1964 and later modifications of the Helsinki declaration (as revised in 2013) on the use of human subjects for research. Written informed consent was obtained from the patient before GENIE cohort registration.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mendall MA, Goggin PM, Molineaux N, et al. Relation of Helicobacter pylori infection and coronary heart disease. Heart 1994;71:437-9. [Crossref] [PubMed]
- Ražuka-Ebela D, Giupponi B, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter 2018;23:e12520. [Crossref] [PubMed]
- Franceschi F, Gasbarrini A, Polyzos SA, et al. Extragastric diseases and Helicobacter pylori. Helicobacter 2015;20:40-6. [Crossref] [PubMed]
- Franceschi F, Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol 2007;21:325-34. [Crossref] [PubMed]
- Roussos A, Tsimpoukas F, Anastasakou E, et al. Helicobacter pylori seroprevalence in patients with chronic bronchitis. J Gastroenterol 2002;37:332-5. [Crossref] [PubMed]
- Siva R, Birring SS, Berry M, et al. Peptic ulceration, Helicobacter pylori seropositivity and chronic obstructive pulmonary disease. Respirology 2013;18:728-31. [Crossref] [PubMed]
- Sze MA, Chen YWR, Tam S, et al. The relationship between Helicobacter pylori seropositivity and COPD. Thorax 2015;70:923-9. [Crossref] [PubMed]
- Fullerton D, Britton JR, Lewis SA, et al. Helicobacter pylori and lung function, asthma, atopy and allergic disease—a population-based cross-sectional study in adults. Int J Epidemiol 2008;38:419-26. [Crossref] [PubMed]
- Lee HY, Kim JW, Lee JK, et al. Association between Helicobacter pylori seropositivity and mild to moderate COPD: clinical implications in an Asian country with a high prevalence of H. pylori. Int J Chron Obstruct Pulmon Dis 2016;11:2055-62. [Crossref] [PubMed]
- Wang F, Liu J, Zhang Y, et al. Association of Helicobacter pylori infection with chronic obstructive pulmonary disease and chronic bronchitis: a meta-analysis of 16 studies. Infect Dis (Lond) 2015;47:597-603. [Crossref] [PubMed]
- Park B, Koo SM, An J, et al. Genome-wide assessment of gene-by-smoking interactions in COPD. Sci Rep 2018;8:9319. [Crossref] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-e88. [Crossref] [PubMed]
- Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog 2016;8:8. [Crossref] [PubMed]
- Pan KF, Zhang L, Gerhard M, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016;65:9-18. [Crossref] [PubMed]
- Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104. [Crossref] [PubMed]
- Moayyedi P, Forman D, Duffett S, et al. The association between Helicobacter pylori infection and adult height. Eur J Epidemiol 2005;20:455-65. [Crossref] [PubMed]
- Dore MP, Pes GM, Sferlazzo G, et al. Role of Helicobacter pylori infection in body height of adult dyspeptic patients. Helicobacter 2016;21:575-80. [Crossref] [PubMed]
- Sood MR, Joshi S, Akobeng AK, et al. Growth in children with Helicobacter pylori infection and dyspepsia. Arch Dis Child 2005;90:1025-8. [Crossref] [PubMed]
- Muhsen K, Goren S, Cohen D. H elicobacter pylori Infection in Early Childhood and Growth at School Age. Helicobacter 2015;20:410-7. [Crossref] [PubMed]
- Kocaoglu C, Ozel A, Cayci M, et al. Effect of long-term Helicobacter pylori infection on growth of children: a cohort study. World J Pediatr 2016;12:196-201. [Crossref] [PubMed]
- Huang RG, Xiao HL, Zhou B, et al. Serum pepsinogen levels are correlated with age, sex and the level of Helicobacter pylori infection in healthy individuals. Am J Med Sci 2016;352:481-6. [PubMed]
- Yang Y, Xiong W, Wang S, et al. Factors associated with detection of Helicobacter pylori in gastric biopsies: a case-control study of 396 biopsies. Appl Immunohistochem Mol Morphol 2018;26:345-50. [PubMed]
- Franceschi F, Zuccalà G, Roccarina D, et al. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol 2014;11:234-42. [Crossref] [PubMed]
- Bravo D, Hoare A, Soto C, et al. Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J Gastroenterol 2018;24:3071-89. [Crossref] [PubMed]
- Mahmood S, Hamid A. Comparison between invasive and noninvasive tests in diagnosis of Helicobacter pylori infection. Pak J Biol Sci 2010;13:509-12. [Crossref] [PubMed]
- Available online: http://oldrf.org/Kr/Home/Download?path=~%2FData%2FPublication%2FDownload%2F%2F84-87_OLD08.pdf&filename=84-87_OLD08.pdf
- Peng YH, Chen CK, Su CH, et al. Increased risk of chronic obstructive pulmonary disease among patients with Helicobacter pylori infection: a population-based cohort study. Clin Respir J 2017;11:558-65. [Crossref] [PubMed]


