
Long-term hydroxychloroquine therapy improves the quality of sleep in patients with primary Sjögren’s syndrome: a real-world study
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic inflammatory autoimmune disease that affects the exocrine glands. Its key features are dry mouth, dry eyes, and other multi-system damage including fatigue, fever, vasculitis, joint pain and kidney injury. The global prevalence rate is about 0.04% to 4.8%, the prevalence in China is 0.29% to 0.77% (1,2). Progress in treating pSS includes biological agents, combination of steroids immunosuppressive agents (1,2). The disease affects middle-aged women aged 40 to 50 years old. In addition to reducing the secretion of exocrine glands, the disease can also cause symptoms, including weakness and pain. These discomforts will eventually lead to a decrease in the quality of life (QoL) of the patient, especially the deterioration of sleep quality, mainly manifested as nighttime sleep disturbance and excessive daytime sleep, and a higher prevalence of obstructive sleep apnea syndrome. Clinical studies have found that sleep disorders are more common in patients with pSS (3,4). The Pittsburgh Sleep Quality Index (PSQI) was used to evaluate patients’ sleep quality in a study, and the researchers found that among Chinese pSS patients, 57.5% of patients have sleep disorders (4). Modern medicine believes that sleep is closely related to many diseases, such as coronary heart disease, hypertension and stroke, and also affects mental and psychological health, including increasing the risk of anxiety, depression, and dementia (5,6). However, it is still unclear what the main mechanism of pSS associated sleep disorders are, which may be related to dry mouth, dry eyes, and weakness. The current research focuses on the influencing factors of sleep disorders in patients with pSS. And there are few studies focused on the effect of different treatments on the sleep quality of these patients.
The main evaluation parameters of most intervention studies look at the function of exocrine glands, and disease activity indexes, including the European League Against Rheumatism Sjögren’s Syndrome Patients Reported Index (ESSPRI). There was no comparison of sleep quality before and after treatment or during follow-up. Hydroxychloroquine (HCQ) has been widely used in rheumatic diseases. Some pSS patients are also receiving HCQ treatment. Studies have shown HCQ can significantly alleviate the symptoms of dry mouth, dry eyes, and joint pain in pSS patients and can also reduce circulating inflammatory factors. Simultaneously, it has excellent safety (7). Whether the patient’s condition improves can also improve sleep quality is still lacking related research. In this study, we evaluated the sleep quality of patients through follow-up and retrospectively analyzed the effects of different treatment methods on patients’ sleep quality.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1380).
Methods
The research subjects included pSS patients who were treated in our hospital from January 2015 to December 2019. According to the inclusion and exclusion criteria, a total of 383 patients were enrolled in the final analysis. Among these patients, 208 patients had poor sleep (poor sleep group, PSG) and 175 patients had good sleep quality (good sleep group, GSG). Inclusion criteria: (I) ≥18 years old; (II) the diagnosis of pSS is confirmed according to China’s Sjögren’s Syndrome Diagnosis and Treatment Guidelines (2) and the American Rheumatology Society’s diagnostic criteria (8); (III) receiving anti-pSS treatment when enrolled. Exclusion criteria: (I) diagnosed with malignant tumor; (II) complicated with severe heart failure; (III) complicated with other diagnosed systemic autoimmune diseases; (IV) complicated with other diseases that affect sleep; (V) subjects who have been diagnosed with mental and mental illness before a diagnosis of pSS; (VI) long-term history of alcoholism; (VII) those who cannot complete the questionnaire survey. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Guangzhou Chest Hospital. and informed consent was taken from all the patients.
Data collection
Enrolled patients were followed up and evaluated. Patients’ clinical information was collected from hospital records and clinic records. The main parameters include age, gender, marital status, education level, employment status, income level, life habits, underlying diseases, and medication. Sleep quality assessment: the PSQI Scale is used to assess the sleep quality of patients in the past month. Each part is scored on a scale of 0–3, with a total score of 21 points. The scale was revised in 1996 by experts, including Liu Xianchen and other experts (9), and the PSQI score >7 was used as the cut-off value for dividing good and poor sleep quality. Both reliability and validity are high, which is suitable for the Chinese population. This study also uses the score >7 points that are judged as sleep disorders. QoL assessment: The World Health Organization Quality of Life Brief Version (WHOQOL-BREF) was used for QoL assessment. The form was simplified by WHOQOL-100 and contained 26 questions in four areas. Evaluation of Sjögren’s Syndrome: The European League Against Rheumatism Sjögren’s Syndrome Patients Reported Index (ESSPRI) was used for evaluation.
Grouping of patients
According to the results of PSQI assessment, patients were divided into good sleep group (GSG) and poor sleep group (PSG). Univariate and multivariate analysis is used to find factors related to sleep disorders. According to whether the patients take HCQ, they are further divided into the HCQ-administered group, and non-administered group and the prognosis and sleep quality of the two groups is compared.
Statistical analysis
SPSS17.0 software was used for statistical processing. Quantitative data were evaluated for normal homogeneity. Data that conformed to the normal distribution were expressed as mean ± standard deviation, and the student t-test was used for comparison between groups; medians were used for non-compliant distribution, and the rank-sum test was used for comparison between groups. Qualitative data are expressed in numerical values and percentages, and comparison between groups is performed using the X2 test or Fisher’s exact test. Logistic univariate and multivariate analysis were used to find factors related to sleep disorders in pSS patients. P<0.05 was considered statistically significant.
Results
Baseline data
From January 1, 2015, to December 31, 2019, 517 patients with pSS were treated in our hospital. According to the inclusion and exclusion criteria, a total of 383 patients were enrolled in the final analysis. The patients ranged in age from 27 to 71 years old, with an average of 47.6±12.8 years old and a disease course of 5.1±2.7 years. There were 23 males (6.0%) and 360 females (94.0%). A total of 208 (54.3%) patients had PSQI ≥7 points (that is, sleep disorders), and a total of 175 (45.7%) patients with PSQI <7 points (good sleep quality). The baseline data of patients are shown in Table 1. There are statistical differences between the two groups in marital status, menopause, education, income, and long-term use of HCQ. Among all patients, 230 patients (60.1%) took HCQ for a long time, and 153 patients (39.9%) did not take HCQ. In this group of patients, the dosage of HCQ is 200–400 mg/day. HCQ treatment duration is from 4 months to 41 months, and the total dose is 24.8 to 371.4 g.

Full table
Factors related to sleep quality in patients with pSS
Univariate analysis showed that divorce, menopause, low income, low education, and long-term use of HCQ and ESSPRI all have a specific relationship with pSS patients’ sleep quality. Further multivariate analysis showed that menopause, low income, long-term use of HCQ, and ESSPRI were associated with sleep quality in patients with pSS. See Table 2 for details.

Full table
The effect of long-term HCQ treatment on the pSS condition and QoL of patients
We conducted a pSS condition assessment on all patients and found the ESSPRI of patients taking HCQ was significantly lower than those who did not (P<0.05). See Table 3 for details. The QoL assessment analysis showed that the QoL of the patients in the long-term HCQ-administrated group was higher than that in the non-administrated group. The specific performance is that the patients in the long-term HCQ-administrated group have better scores in the physiological, psychological, social relationship, and environmental fields than the non-administrated group. Further analysis found that the QoL of patients with sleep quality disorders was lower than patients without sleep disorders.
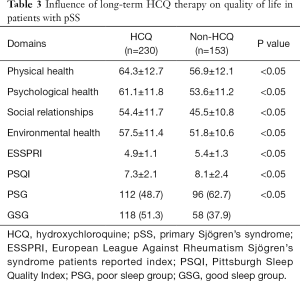
Full table
Discussion
In this study, we conducted a survey and analysis of pSS patients in the real world and found that compared with patients who did not take HCQ in the past three months, patients who took HCQ for a long time not only significantly improved the condition index ESSPRI of pSS but also decrease their sleep quality index PSQI. The WHOQOL-100 score of QoL in patients with long-term HCQ therapy was significantly higher than that in patients without HCQ therapy in the past three months. With this result, we believe that long-term use of HCQ cannot only improve the pSS condition, but also significantly improve the sleep quality of pSS patients, and may lead to an improvement in the QoL. According to the literature search, this is the first real-world study on the sleep quality of pSS patients taking long-term HCQ.
In addition to involving the secretion of salivary glands and lacrimal glands, pSS also has some extra-glandular manifestations, including weakness, pain, depression, etc. (10). Decreased sleep quality is a widespread problem in patients with pSS. The main reason is that pSS affects exocrine glands, including salivary glands and lacrimal glands, to cause dry mouth and dry eyes. It also affects joint muscles and causes pain in the motor system. It is also a secondary cause of psychological problems caused by diseases (3,11). If the condition of pSS is controlled and the secretory function of the exocrine glands is improved, the quality of sleep will often be improved (12,13) in some patients. When the severity of the patient’s condition is different, and the organs and tissues involved are different, there are often different treatment options, including simple symptomatic treatment, immunosuppressive treatment and device-assisted treatment, which can be selected according to the specific situation of the patient (14). However, is there any difference that exists between different medications to improve sleep quality? There has been no explicit study to discuss this issue. Secondly, besides, to improve the control of pSS, is there any other possibility to improve sleep? Also, because there are many reasons for the decline in sleep quality, some symptomatic methods may improve sleep. For example, Cognitive Behavioral Therapy for Insomnia (CBT-I) is aimed at patients with underlying diseases, especially some patients with long-term chronic pain or other discomfort (15,16). Symptomatic relief from dry mouth and dry eyes may also help improve sleep quality, but this method may be more limited to mild patients, and for those with more severe dry mouth and dry eye symptoms, it may not be effective (17,18) as usual. For patients with limb pain, proper administration of analgesic drugs can effectively relieve pain, especially the reduction of pain at night, which is conducive to sleep improvement (19,20). When the patient develops nocturnal obstructive apnea syndrome, continuous positive airway pressure may improve the patient’s breathing, thereby improving sleep quality and QoL (21). In terms of systemic treatment, currently mainly used drugs include glucocorticoids, immunosuppressants and bio-targeted drugs, but the impact of these drugs on the sleep quality of pSS patients is currently unclear. HCQ is an established drug widely used in rheumatic diseases in recent years. Several studies have supported HCQ in systemic lupus erythematosus (22), systemic vasculitis (23) and rheumatoid arthritis (24), and other diseases, and there are still some studies suggesting that HCQ is relatively safe for long-term use, especially at a lower dosage (25,26). However, many studies have found that HCQ has certain retinal toxicity (27) and cardiotoxicity (28). During HCQ therapy, these toxicities should be closely monitored. When cardiotoxicity occurs, it can recover by itself after stopping the drug in time (29). There are few reports on the effects of long-term use of HCQ on sleep. Earlier, Manzo et al. reported an elderly female patient who experienced psychomotor agitation after taking HCQ. The authors analyzed that HCQ can cause irritability, nervousness, emotional changes and nightmares (30). Once the patient occurs, spiritual agitation and sleep disturbances can hurt family members living together, and further reduce the QoL through various mechanisms (31). However, there is no relationship between this phenomenon and the sleep of such patients. Since there are few confirmed cases, there is no relevant research.
The results of this study show that compared with patients who do not use HCQ, the risk of sleep disturbance in patients who take HCQ for a long time is significantly reduced. The average sleep quality of patients with long-term HCQ therapy is better than those who do not take it, and this effect is independent of the improvement of the disease condition. However, few studies focus on the relationship between HCQ and sleep quality. It is inconsistent with the earlier research results (30,32). As for a reason, there are few cases of mental side effects of HCQ that are reported until now. The relationship between this side effect and sleep is currently unclear. Our research shows that the pSS condition of this group of patients is effectively controlled, and the symptoms of dry mouth and dry eyes are significantly alleviated. During the follow-up, no patients showed typical agitation and various levels of mental excitement. Through a literature search, only 4 cases of HCQ related to psychiatric symptoms have been reported in China. Foreign scholars have integrated relevant literature and believe that the main risk factors for mental changes in patients taking HCQ include drug interactions, alcohol consumption during medication, family history of mental illness, women, and concurrent use of low-dose glucocorticoids (32). When psychiatric symptoms occur, symptomatic treatment or withdrawal of HCQ can effectively reverse this side effect.
In this study, the long-term daily dose of HCQ was 200–400 mg/d. The duration of HCQ therapy was 4 to 41 months; the total dose was 24.8–371.4 g, which was low. During the period of medication, these patients used fewer types of drugs, with an average of 2–3 types. No obvious drug interaction was found, and only a few patients had drunk a small amount of alcohol during the period of HCQ therapy. There was no clear family history of mental illness. Some patients were taking small doses of prednisone together, so no clear mental changes were found in this group of patients. Therefore, the possibility of sleep quality decrease due to mental changes was slight in this study. According to results from previous studies and our research, HCQ caused mental changes only in specific individual patients and did not affect most of the patient’s sleep quality. Secondly, the selective use of HCQ can significantly reduce the incidence of mental disorders.
Concurrently, no new onset eye diseases or diagnosed retinopathy were reported in all patients. The 2008 American College of Rheumatology recommended that when the total dose of HCQ exceeds 1,000 g, the risk of retinal toxicity increases significantly (33). Most of the high-risk factors of retinal toxicity have been excluded from the prescription of HCQ in this group of patients, including severe liver and kidney disease, advanced age, existing retinal or macular degeneration, cataracts, and significant obesity. However, with the prolonged use of drugs and the increase of cumulative doses, the risk of retinal toxicity of patients also increases. Therefore, it is necessary to conduct regular retinal examinations for patients who receive long-term and high dosage HCQ therapy. In terms of cardiotoxicity, there are no clearly diagnosed cases in this group of patients. Individual patients with reduced cardiac function have basic coronary heart disease, and HCQ has not aggravated related symptoms.
Limitations of this study: this study did not further evaluate the psychiatric scale of patients with sleep disorders. Only some patients were treated symptomatically. Although it is indirectly proved that long-term use of HCQ can improve the sleep quality of pSS patients, the study is not in-depth enough, failing to analyze the specific mechanism of HCQ to improve the sleep of patients with pSS. And, because this is a retrospective study, we didn’t collet blood sample at baseline for related cytokines test. Future research can be targeted for relevant examinations, including sleep polysomnography, psychiatric scale evaluation, some cytokines and can also assess the effects of related drugs on sleep-related factors, including whether HCQ affects depression in pSS patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1380
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1380
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1380). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Guangzhou Chest Hospital and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cafaro G, Croia C, Argyropoulou OD, et al. One year in review 2019: Sjögren's syndrome. Clin Exp Rheumatol 2019;37 Suppl 118:3-15. [PubMed]
- Rheumatology Branch of Chinese Medical Association. Guideline for diagnosis and treatment of primary Sjögren's syndrome. Chin J Rheumatol 2010;14:766-7.
- Hackett KL, Gotts ZM, Ellis J, et al. An investigation into the prevalence of sleep disturbances in primary Sjögren's syndrome: a systematic review of the literature. Rheumatology (Oxford) 2017;56:570-80. [PubMed]
- Cui Y, Li J, Li L, et al. Prevalence, correlates, and impact of sleep disturbance in Chinese patients with primary Sjögren's syndrome. Int J Rheum Dis 2020;23:367-73. [Crossref] [PubMed]
- Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, et al. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep 2011;34:1487-92. [Crossref] [PubMed]
- Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013;70:587-93. [Crossref] [PubMed]
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren's Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol 2017;69:35-45. [Crossref] [PubMed]
- Liu XC, Tang MQ, Hu L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry 1996;29:103-7.
- Bodewes ILA, Gottenberg JE, van Helden-Meeuwsen CG, et al. Hydroxychloroquine treatment downregulates systemic interferon activation in primary Sjögren's syndrome in the JOQUER randomized trial. Rheumatology (Oxford) 2020;59:107-11. [Crossref] [PubMed]
- Karageorgas T, Fragioudaki S, Nezos A, et al. Fatigue in Primary Sjögren's Syndrome: Clinical, Laboratory, Psychometric, and Biologic Associations. Arthritis Care Res (Hoboken) 2016;68:123-31. [Crossref] [PubMed]
- Westhoff G, Dorner T, Zink A. Fatigue and depression predict physician visits and work disability in women with primary Sjogren’s syndrome: results from a cohort study. Rheumatology 2012;51:262-9. [Crossref] [PubMed]
- Price EJ, Rauz S, Tappuni AR, et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren's Syndrome. Rheumatology (Oxford) 2017;56:e24-48. [Crossref] [PubMed]
- Carsons SE, Vivino FB, Parke A, et al. Treatment Guidelines for Rheumatologic Manifestations of Sjögren's Syndrome: Use of Biologic Agents, Management of Fatigue, and Inflammatory Musculoskeletal Pain. Arthritis Care Res (Hoboken) 2017;69:517-27. [Crossref] [PubMed]
- Raglan GB, Swanson LM, Arnedt JT. Cognitive Behavioral Therapy for Insomnia in Patients with Medical and Psychiatric Comorbidities. Sleep Med Clin 2019;14:167-75. [Crossref] [PubMed]
- Pigeon WR. Treatment of adult insomnia with cognitive-behavioral therapy. J Clin Psychol 2010;66:1148-60. [Crossref] [PubMed]
- Wu AJ. Optimizing dry mouth treatment for individuals with Sjögren's syndrome. Rheum Dis Clin North Am 2008;34:1001-10. [Crossref] [PubMed]
- Ghosh P, Lazar AA, Ryan WR, et al. A feasibility and efficacy trial of a hand-held humidification device in patients undergoing radiotherapy for head and neck cancer. Support Care Cancer 2017;25:2611-8. [Crossref] [PubMed]
- Omdal R, Mellgren SI, Norheim KB. Pain and fatigue in primary Sjögren's syndrome. Rheumatology (Oxford) 2019. [Crossref] [PubMed]
- Vitali C, Del Papa N. Pain in primary Sjögren's syndrome. Best Pract Res Clin Rheumatol 2015;29:63-70. [Crossref] [PubMed]
- Usmani ZA, Hlavac M, Rischmueller M, et al. Sleep disordered breathing in patients with primary Sjögren's syndrome: a group controlled study. Sleep Med 2012;13:1066-70. [Crossref] [PubMed]
- Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol 2016;12:456-71. [Crossref] [PubMed]
- Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf 2017;16:411-9. [Crossref] [PubMed]
- Casian A, Sangle SR, D'Cruz DP. New use for an old treatment: Hydroxychloroquine as a potential treatment for systemic vasculitis. Autoimmun Rev 2018;17:660-4. [Crossref] [PubMed]
- Rynes RI. Hydroxychloroquine treatment of rheumatoid arthritis. Am J Med 1988;85:18-22. [Crossref] [PubMed]
- Jorge A, Ung C, Young LH, et al. Hydroxychloroquine retinopathy - implications of research advances for rheumatology care. Nat Rev Rheumatol 2018;14:693-703. [Crossref] [PubMed]
- Dogar MU, Shah NN, Ishtiaq S, et al. Hydroxychloroquine-induced restrictive cardiomyopathy: a case report. Postgrad Med J 2018;94:185-6. [Crossref] [PubMed]
- Shinjo SK, Bonfá E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010;62:855-62. [Crossref] [PubMed]
- An Y, He YL, Jia Y, et al. Prospective study on the long-term ocular toxicity of hydroxychloroquine in rheumatic diseases. Chin J Rheumatol 2009;13:178-80.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762-84. [Crossref] [PubMed]
- Yogasundaram H, Hung W, Paterson ID, et al. Chloroquine-induced cardiomyopathy: a reversible cause of heart failure. ESC Heart Fail 2018;5:372-5. [Crossref] [PubMed]
- Mascolo A, Berrino PM, Gareri P, et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology 2018;26:1141-9. [Crossref] [PubMed]
- Manzo C, Gareri P, Castagna A. Psychomotor Agitation Following Treatment with Hydroxychloroquine. Drug Saf Case Rep 2017;4:6. [Crossref] [PubMed]
- Livingston G, Barber J, Marston L, et al. Clinical and cost-effectiveness of the Managing Agitation and Raising Quality of Life (MARQUE) intervention for agitation in people with dementia in care homes: a single-blind, cluster-randomised controlled trial. Lancet Psychiatry 2019;6:293-304. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


