Sonography monitoring of thyroid morphology and function in patients with metastatic renal cell carcinoma treated with targeted drugs
Introduction
Renal cell carcinoma (RCC) is a heterogeneous group of cancers that is derived from renal tubular epithelial cells. RCC is one of the most common cancers worldwide, accounting for 2% of all adult malignant tumors (1,2). Furthermore, the incidence rate of RCC has risen in the world over the past few decades (2). Clear cell RCC (ccRCC) is the most common histologic type of RCC, accounting for 75% of all primary RCCs. The pathogenesis of RCC remains unclear according to present research; however, it is known to be closely related to genetics, immunity, smoking, obesity, and hypertension (3). Unfortunately, there are no apparent symptoms in the early stage of RCC, so most patients are initially diagnosed with metastasis. The prognosis of advanced RCC is poor, and the 5-year survival rates of stage III and stage IV RCC were found to be 50% and 10%, respectively (3,4). Science and medical technology advancements have allowed for a deeper understanding of RCC pathogenesis. Merinopoulou et al. found that RCC is related to the rapid activation and expansion of hypoxia-inducible factors caused by an abnormal expression of the Von Hippel-Lindau (VHL) gene causing vascular endothelial growth factor (VEGF), TGF-α, and platelet-derived growth factor-β overexpression which in turn promote the survival, migration, diffusion, and proliferation of RCC cells (5). At present, targeted therapy is the primary method of therapy for metastatic mRCC, mostly ccRCC. In 2006, the National Comprehensive Cancer Network (NCCN) clinical practice guide for renal carcinoma in the United States recommended vascular endothelial growth factor (VEGF) inhibitors (sorafenib, etc.) as the first-line drug for the treatment of mRCC (6). Furthermore, thyroid dysfunction is one of the most common adverse reactions of targeted treatment (7). This study aimed to investigate the changes of thyroid volume in patients with targeted drugs and to understand if the changes in thyroid volume monitored by ultrasound can predict the risk of hypothyroidism in these patients.
Methods
Subjects
From September 2016 to April 2019, data from 52 patients with mRCC were collected in our hospital. Finally, 37 patients were enrolled in our study (26 males and 11 females, aged 36–76 years). Among them, 19 patients were treated with sunitinib and 18 patients with sorafenib.
Inclusion criteria
The inclusion criteria for patients were as follows:
- newly diagnosed with mRCC (without any treatment history) by pathology and imaging;
- aged ≤80 years old;
- normal thyroid function and no history of thyroid hormone treatment;
- no other treatment plans to be received after targeted treatment;
- the clinical data could be collected through our hospital's hospital information system (HIS).
Exclusion criteria
The exclusion criteria for patients were as follows:
- any other major diseases;
- treatment with more than 1 kind of targeted drug;
- cachexia or multiple organ failure;
- unable to be followed up with on-time or with incomplete medical records;
- total and subtotal thyroidectomy, irregular shape of the thyroid glands.
Equipment
The equipment used in this study was the GE Logiq E9 (GE Healthcare, Milwaukee, WI, USA) color doppler ultrasonic diagnostic instrument and linear array probe, at a probe frequency of 5–10 MHz.
Calculation method of thyroid volume and volume change rate
The upper-lower diameter (length-diameter/major-axis) was measured along the largest cross-section of the long-axis of the thyroid-side lobe. The left and right diameters (width diameters) and anteroposterior diameters (thickness diameters) were measured at the maximum section of the transverse axis of the lateral lobe of the thyroid gland. The same method was used to measure the diameters of the isthmus.
The volume of the bilateral lobes and the isthmus was calculated using the elliptical formula: V = π/6 × length–diameter × transverse diameter × thick diameter. Next, the thyroid volume was obtained by adding the volume of the 2 bilateral lobes and isthmus.
The thyroid volume change rate was calculated as follows: thyroid volume change rate = (thyroid volume before treatment − Nth month thyroid volume after treatment)/thyroid volume before treatment.
Detection of thyroid function related hormones
Thyroid hormones were examined just before treatment. The hormones were additionally measured on the 1st, 2nd, 3rd, 4th, 5th, 6th, 9th, and 12th months after treatment. Fasting venous blood of 3 mL was taken before 10:00 a.m., and the concentration of FT3, FT4, and thyroid-stimulating hormone (TSH) in serum was determined using Roche's method. The concentration of TSH was used as the criterion for the diagnosis of thyroid dysfunction.
Clinical hypothyroidism diagnostic criteria were as follows: TSH >4.2 IU/mL, FT3 <3.1 pmol/L, and FT4 <12 pmol/L. Subclinical hypothyroidism diagnostic criteria were as follows: TSH >4.2 IU/mL, FT3: 3.1–6.8 pmol/L, and FT4: 12.0–22.0 pmol/L.
Statistical methods
SPSS 24 statistical analysis software was used to analyze and measure the data for the normal tests. The non-normal data were described by the quartile range (QR). T-tests were used for the comparison of the normal data. The rank-sum test was used for the comparison of the non-normal data. Rank-sum tests and repeated measurement variance analysis were used to compare the thyroid volume change rate between the hypothyroidism group and the non-hypothyroidism group. The diagnostic ability of the thyroid volume change rate on thyroid function was calculated by receiver operating characteristic curve (ROC).
Results
Clinical data
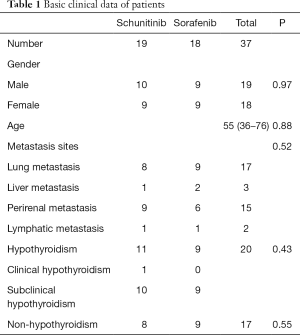
Data from 52 patients with mRCC from September 2016 to April 2019 were collected; 37 cases met the inclusion criteria (19 cases were treated with sunitinib, and 18 cases were treated with sorafenib. In total, 26 males and 11 females, aged 36–76 years old were included. The primary clinical data of the 37 patients are shown in Table 1. There were no significant differences in the patients’ sex, age, metastatic places, and incidence rate of hypothyroidism between the sunitinib and sorafenib groups (P>0.05).
Full table
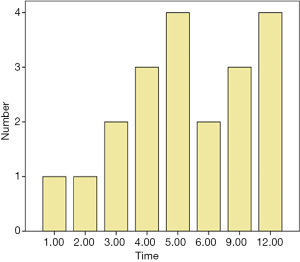
Before targeted treatment, the serum concentrations of TSH, FT3, and FT4 in all patients were normal, and the hormone levels of 20 patients became abnormal during the 12-month follow-up. There was 1 case of clinical hypothyroidism (TSH =9.7 IU/mL, FT3 =1.6 pmol/L, FT4 =6.6 pmol/L), and 19 cases of subclinical hypothyroidism (TSH >4.2 IU/mL). No patients received thyroid hormone replacement. During the follow-up process, all 20 patients’ thyroid function recovered gradually. The timing of the occurrence of hypothyroidism is shown in Figure 1. The average time of the occurrence of hypothyroidism was 6.4 months. No hypothyroidism was found in the remaining 17 patients, and no hyperthyroidism was found in any patient.
Changes in thyroid function-related hormone levels
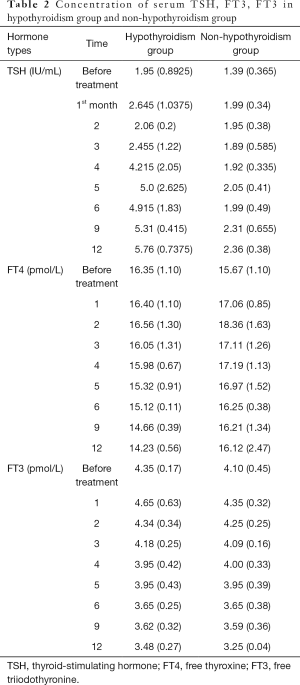
There was no significant difference in serum TSH, FT3, FT3 levels between the sunitinib group and the sorafenib group after treatment. The repeated measurement variance analysis was F =0.01, P=0.976; F =0.006, P=0.937; and F =0.008, P=0.928. The concentration of FT3, FT4, and TSH levels in serum before and after treatment on the 1st, 2nd, 3rd, 4th, 5th, 6th, 9th, and 12th months in the hypothyroidism group and the non-hypothyroidism group are shown in Table 2. There were no significant differences in serum concentration of TSH, FT3, FT3 between the hypothyroidism group and the non-hypothyroidism group before treatment. There was a significant difference in the concentration of TSH between the hypothyroidism and non-hypothyroidism groups after treatment (repeated measurement variance analysis: F =41.394, P=0.000). There was no significant difference in serum FT3 and FT4 levels between the hypothyroidism and non-hypothyroidism groups after treatment (repeated measurement variance analysis: F =0.006, P=0.937; F =0.437, P=0.703). There was no interaction between the time of medication and the change of serum concentration of TSH, FT3, and FT3 levels (repeated measurement variance analysis: F =0.975, P=0.826; F =1.504, P=0.167; F =0.407, P=0.898).
Full table
Thyroid volume and volume change rate monitored by ultrasound after targeted treatment
There was no significant difference in the rate of change for thyroid volume between the sunitinib group and the sorafenib group after treatment (repeated measurement variance analysis: F =0.873, P=0.356).
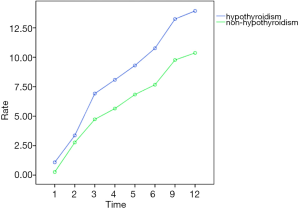
The thyroid volume and the rate of volume change before and after treatment at the 1st, 2nd, 3rd, 4th, 5th, 6th, 9th, and 12th months in the hypothyroidism and non-hypothyroidism groups are shown in Table 3. There were no significant differences in thyroid volume between the hypothyroidism and non-hypothyroidism group before treatment (P>0.05). There was a significant difference in the rate of thyroid volume change between the hypothyroidism and non- hypothyroidism group after treatment (F =490.804, P=0.000). There was a correlation between the medication time and the thyroid volume change rate (F =20.798, P=0.000), which meant that the rate of thyroid volume change increased gradually with prolonged treatment times. The rate of volume change after targeted treatment for the hypothyroidism and non-hypothyroidism groups is shown in Figures 2,3. The curves of the time-thyroid volume change rate in the hypothyroidism group and the non-hypothyroidism group are shown in Figure 4.

Full table
Diagnostic ability of sonography monitoring of thyroid volume for thyroid function
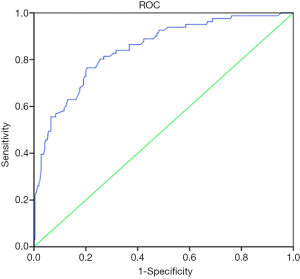
The ROC was drawn by taking the rate of thyroid volume change post targeted drug usage as a diagnostic index for thyroid dysfunction (Figure 5). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for hypothyroidism when the thyroid volume change rate of 8.5% was taken as a cut-off are shown in Table 4. The area under the curve (AUC) was 0.845.

Full table
Discussion
At present, the commonly used target drugs for mRCC are sunitinib and sorafenib. Sunitinib and sorafenib are orally ingested small-molecule tyrosine kinase receptor inhibitors (TKIs). Receptors of tyrosine kinases (RTKs) can be seen as transmembrane glycoproteins that can trigger the creation of intracellular signal transduction pathways after binding to ligands. These creations eventually lead to the transcription of genes such as cell proliferation, angiogenesis, adhesion, and movement (8). The antitumor activity of sunitinib and sorafenib is achieved by a precise inhibition of the related RTKs including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and stem cell factor receptor, etc. These inhibitions induce tumor cell apoptosis and inhibit angiogenesis (9).
It is hypothesized that TKIs act directly on thyroid follicular cells and block the interactions between vascular endothelial growth factor receptors (VEGFRs) and VEGFs. Furthermore, VEGFR-2 is the key to maintaining the integrity of thyroid vessels. When TKIs are blocked in the VEGF-A-VEGFR-2 axis, it not only affects the growth and development of the thyroid vessels but also affects the proliferation of thyroid follicles (10), which leads to hypothyroidism. It is reported in the literature that there is a high incidence rate (sunitinib 10–85%; sorafenib 6.3–27%) of hypothyroidism caused by targeted drugs (11). In our research, the incidence rate of clinical hypothyroidism was lower (1/37, 2.7%), and there was a higher incidence rate of subclinical hypothyroidism (19/37, 51.4%).
In this study, the thyroid volume of patients with targeted drugs showed a decreasing trend. Furthermore, the change in thyroid volume was more significant in the hypothyroidism group. On the 12th month after treatment, the volumes of the thyroid group and non-hypothyroidism group decreased by 14.15% and 10.60%, respectively. Some studies (12-14) have shown that thyroid dysfunction is caused by TKIs, which may be due to the inhibition of VEGFR2-induced degradation of the capillary network in the glands. These dysfunctions mainly manifest in poor vascular structure, fibrin deposition, and blood flow stagnation, followed by the loss of vascular endothelial cells and a decrease of thyroid capillary density, which can be as high as 68%. This mechanism may be one of the reasons for the significant decrease in thyroid volume in patients with mRCC taking targeted drugs. With the prolongation of treatment time, the more serious the damage to the thyroid vascular structure becomes, and the smaller the volume of thyroid gland is.
Ultrasonic monitoring of thyroid volume is simple, noninvasive, and easy to repeat. This study explored the feasibility of ultrasonic monitoring of thyroid function in patients with mRCC taking targeted drugs. After targeted therapy, the thyroid volume change rate in hypothyroidism group (n=20) on the 1st, 2nd, 3rd, 4th, 5th, 6th, 9th, and 12th month was 0.84 (0.47)%, 3.36 (1.34)%, 6.95 (1.42)%, 8.57 (1.66)%, 9.15 (0.55)%, 10.40 (1.12)%, 13.30 (1.53), and 14.15(1.85)%, respectively. For the non-hypothyroidism group (n=17), the thyroid volume change rate was 0.25 (0.11)%, 3.00 (1.81)%, 3.9 (0.75)%, 5.09 (1.81)%, 6.72 (1.81)%, 6.90 (1.35)%, 9.90 (1.35)%, and 10.60 (1.79)%, respectively. There was a significant difference in the rate of change in thyroid volume between the 2 groups (P<0.05). The results showed that ultrasound examinations could indirectly reflect hypothyroidism and reduce the frequency of having to take blood samples in patients with advanced tumors. In this study, we attempted to judge the occurrence of hypothyroidism using thyroid volume change rate. ROC showed that good diagnostic ability was achieved when the volume change rate was 8.5%
Some limitations to this study should also be addressed. Due to the prohibitive price of targeted therapy, long-term drug resistance, or a lack of regular follow-up, the sample size of this study was small. During the research period, thyroid function recovered in patients with hypothyroidism, but no corresponding volume changes were observed by ultrasound, which may be related to the lack of follow-up time in this study. These specific mechanisms need to be further investigated.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-524
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-524). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is a routine examination of renal cancer patients treated with targeted drugs by ultrasound equipment, and the data are recorded during examination and follow-up. This process does not need to be ethics approval, in addition, this study is non-invasive and there is no any ethical violation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- James JH, Mark PP, Sabina S, et al. Renal Cell Carcinoma. Nat Rev Dis Primers 2017;3:17009. [PubMed]
- Petejova N, Martinek A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016;160:183-94. [Crossref] [PubMed]
- Quinn DI, Zhang T, Gurney H, et al. Phase 3, randomized, double-blind trial of pembrolizumab in the adjuvant treatment of renal cell carcinoma (RCC): KEYNOTE-564. J Clin Oncol 2018;36:S712. [Crossref]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Merinopoulou E, Ramagopalan S, Malcolm B, et al. Methods for Extracting Treatment Patterns for Renal Cell Carcinoma (RCC) from Social Media (SM) Forums Using Natural Language Processing (NLP) and Machine Learning (ML). Value Health 2017;20:A402. [Crossref]
- Jun G, Ma JH, Sun Y, et al. Chinese guideline on the management of renal cell carcinoma(2015 edition). Ann Trasnsl Med 2015;3:279.
- Fischer S, Gillessen S, Rothermundt C. Sequence of treatment in locally advanced and metastatic renal cell carcinoma. Transl Androl Urol 2015;4:310-25. [PubMed]
- Sabatier R, Chrisofos M, Safioleas M, et al. Could thyroid dysfunction influence outcome in sunitinib-treated metastatic renal cell carcinoma? Ann Oncol 2012;23:714-21. [Crossref] [PubMed]
- Lechner MG, Vyas CM, Hamnvik OR, et al. Risk Factors for New Hypothyroidism During Tyrosine Kinase Inhibitor Therapy in Advanced Nonthyroidal Cancer Patients. Thyroid 2018.28:437-44.
- Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol 2020;12:1758835920907504. [Crossref] [PubMed]
- Kotani H, Adachi Y, Kitai H, et al. Distinct dependencies on receptor tyrosine kinases in the regulation of MAPK signaling between BRAF V600E and non-V600E mutant lung cancers. Oncogene 2018;37:1775-87. [Crossref] [PubMed]
- Clemons J, Gao D, Naam M, et al. Thyroid dysfunction in patients treated with sunitinib or sorafenib. Clin Genitourin Cancer 2012;10:225-31. [Crossref] [PubMed]
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015.4:560-75.
- Burge M, Semira C, Lee B, et al. Previous Bevacizumab and Efficacy of Later Anti-Epidermal Growth Factor Receptor Antibodies in Metastatic Colorectal Cancer: Results From a Large International Registry. Clin Colorectal Cancer 2018;17:e593-9. [Crossref] [PubMed]