
Lung ultrasound and diaphragmatic excursion assessment for evaluating perioperative atelectasis and aeration loss during video-assisted thoracic surgery: a feasibility study
Introduction
The use of one-lung ventilation (OLV) is widely applied in video-assisted thoracoscopic surgery (VATS) to achieve optimal surgical conditions (1). Lung-protective strategies such as lowering the tidal volume (Vt), lowering the value of inspired oxygen (FiO2), recruitment maneuvers, and the moderate positive end-expiratory pressure (PEEP) has reduced postoperative respiratory complications significantly (2,3). Unfortunately, atelectasis is a common phenomenon during general anesthesia and intrathoracic surgery (4). Meanwhile, thoracic surgery causes unusual weaknesses of the diaphragm’s muscle fibers, and one of the major components of total respiration is diaphragm movement (5). Thus, impairment of diaphragm muscle fibers significantly increases postoperative pulmonary complications (6,7).
The use of bedside lung ultrasound (LUS) has been growing rapidly as a method for the diagnosis of respiratory diseases in the intensive care unit (ICU), operating room (OR), and emergency department due to its lack of radiation, high accuracy, repeatability, portability, and noninvasiveness (8,9). LUS can quickly detect atelectasis (sensitivity 87.7%, specificity 92.1%, accuracy 90.8%) (10), pleural effusion (sensitivity 100%, specificity 100%, accuracy 100%) (11), pneumonia (sensitivity 88%, specificity 86%) (12), and pneumothorax (sensitivity 100%, specificity 82%) (13). Ultrasound assessments of diaphragmatic excursions are correlated with vital capacity (5). Furthermore, the use of LUS is straightforward and safe to use while simultaneously enhancing the specificity and sensitivity of phrenic nerve activity measurement (14). It has also been found to be useful for the serial assessment of diaphragmatic function and motion (sensitivity 91%, specificity 91%) (15).
Although it has been deemed necessary to use LUS daily in the postoperative period to enhance recovery after thoracic surgery (16), there have been no studies on LUS that continuously investigate atelectasis and aeration changes during the perioperative period of VATS. We hypothesized that LUS would allow for the diagnosis of pulmonary complications, especially atelectasis during VATS. Furthermore, it would be possible to use LUS to track and monitor changes in lung aeration. Additionally, we hypothesized that diaphragmatic excursion assessments by ultrasonography could also evaluate aeration loss after VATS. This study primarily aimed to investigate the feasibility of bedside LUS in the perioperative period of VATS and to diagnose perioperative atelectasis. The secondary aim was to investigate the use of continuous evaluation of perioperative lung aeration changes during VATS through LUS examination and diaphragmatic excursion assessment. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-19-595b).
Methods
Patients
The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the institutional review board of the Second Affiliated Hospital of Zhejiang University (I2018001364, 2018/12/05) and registered at ClinicalTrials.gov (no. NCT03802868) and informed consent was taken from all the patients. Consecutive adult patients scheduled for elective video-assisted thoracoscopic lung surgery or mediastinal tumor resection were recruited. Exclusion criteria included the following: noncooperation or delirium after extubation; a body mass index (BMI) of ≥40 kg/m2; a history of respiratory infection; chronic obstructive pulmonary disease or chronic heart disease, history of brachial plexus nerve blockage or general anesthesia within 2 weeks of surgery; previous thoracic procedures (e.g., thoracotomy, thoracoscopy or thoracic drain). Patients were not included for further data analysis in cases of conversion to open thoracotomy, OLV failure (failure to maintain oxygenation and need to convert to two-lung ventilation, TLV), or postoperative admission to the ICU.
Anesthesia protocol
All enrolled patients were pre-oxygenated with 100% oxygen for 3 minutes and received intravenous administration of midazolam 0.03–0.05 mg/kg, sufentanil 0.4–0.6 µg/kg, and etomidate 0.2–0.3 mg/kg for anesthesia induction. Endotracheal intubation was facilitated with rocuronium 0.6–1.0 mg/kg. The proper double-lumen endotracheal tube was inserted to perform the OLV. Anesthesia was maintained with a continuous intravenous infusion of remifentanil and propofol. Supplemental cisatracurium was provided for adequate muscle relaxation (no more than 1 twitch on the train-of-four) when needed. The depth of anesthesia monitoring was completed by the bispectral index (BIS) with an appropriate value of 40–60. Mechanical ventilation with the volume-controlled modes was used for each participant. The OLV parameters were set as a Vt of 5–6 mL/kg, a respiratory rate of 12–14 breaths/min adjusted to sustain an end-tidal carbon dioxide partial pressure (PETCO2) of 35–45 mmHg, and an FiO2 of 0.6–0.8 with a PEEP of 5 cmH2O to maintain a peak airway pressure of less than 30 cmH2O. Lung resection was performed in the lateral position, whereas mediastinal tumor resection was performed in the supine position. Before closing the chest, an alveolar recruitment maneuver set the peak inspiratory pressure limit to 45 cmH2O. Next, a respiratory rate of 6 breaths/min and inspiratory-to-expiratory ratio (I:E) of 1:1 was induced by the anesthesiologist and then returned to pre-recruitment values. The complete expansion of the collapsed lung was directly visualized by the surgeon, and then the OLV was converted to TLV until extubation. The surgeon routinely placed a chest tube attached to a water-sealed bottle at −20 cmH2O suction to drain any subsequent fluid and/or air leakage. Analgesic therapy at the incision site was accomplished with ropivacaine (0.375%, 10 mL) through an intercostal peripheral nerve blockade in each patient. Postoperative pain therapy was implemented by continuous patient-controlled intravenous analgesia for 48 hours. In the post-anesthesia care unit (PACU), mechanical ventilation with the same set of pre-recruitment parameters were used for all patients. Neostigmine was used for the reversal of neuromuscular blocking before extubation in all patients. Patients inhaled oxygen through a mask at 5–6 L/min for at least 15 minutes after extubation.
LUS examination
LUS imaging was performed by 2 trained anesthesiologists (Chen X and Na S, both with at least 1 year of ultrasound training) using a 2- to 5-MHz convex probe in an ultrasound device (Mindray, Guangdong, China). Images were acquired at 4 pre-established timepoints: before anesthesia induction (the timepoint A), 5 minutes after intubation (the timepoint B), at the end of the surgery (the timepoint C), and 15 minutes after extubation (the timepoint D). Each hemithorax was divided into 6 quadrants by anterior and posterior axillary lines, and axial lines (superoanterior, inferoanterior, superolateral, inferolateral, superoposterior, and inferoposterior quadrants) (Figure 1) (17). With the patient in the supine position, the anterior and lateral quadrants were examined.
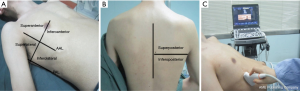
Meanwhile, the examinations of the posterior quadrants were accomplished with the patient slightly turned to the lateral position. The ultrasound probe was moved horizontally along the intercostal spaces in each quadrant. The following data were recorded for each scan: the presence of A lines, the amount or coalescence of B lines, and the number of subpleural consolidations. Once atelectasis was detected at timepoint D, the patient was transported to the Radiology Department for a thoracic computed tomography (CT) scan within 1 hour of detection. Those who did not have any atelectasis discovered on the LUS were transported to their respective wards without any additional investigations except for a routine chest X-ray (CXR) check on postoperative day 1 (Figure 2). A complete ultrasound examination needed a mean duration time of 5 to 10 minutes. For the characterization of the regions, a recorded video of the worst abnormality for each quadrant was analyzed offline by the two anesthesiologists mentioned above (Chen X and Na S). In the cases of disagreement, a third anesthesiologist (Kai S, with 3 years of ultrasound experience) reviewed the uncertain images and made the final diagnosis.
Lung ultrasonography scores
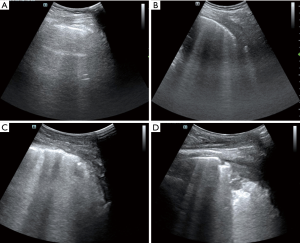
The LUS scores were used to assess aeration loss by retrieving the sum of the score values from 0 to 3 for each quadrant, with a higher grade indicating more severe aeration loss, and no scores being applicable for pneumothorax. The scoring was defined as follows: 0= normal lung with sliding pleura and equidistant A lines parallel to the smooth pleural line; 1= moderate aeration loss and no less than 3 scattered B lines deriving from the pleural line; 2= severe aeration loss and irregular pleural line with coalescent B lines; 3= complete aeration loss and a tissue-like pattern or subpleural consolidation (Figure 3) (10,18). Atelectasis was diagnosed with juxta-pleural hypoechoic consolidations using bright echogenic static air bronchograms or a tissue-like pattern on ultrasonography as a model (Figure 3D) (19). Pleural effusion was described as an anechoic space between the visceral and parietal pleura that also fluctuated with respiratory movement (20). A maximal intrapleural distance of less than 15 mm on ultrasonography was defined as a small effusion (21). Pneumothorax was diagnosed via a combination of the visualization of the “bar code” sign on the M-mode, the lung point, and the absence of lung sliding (22-24). Meanwhile, the presence of lung sliding could help to exclude the diagnosis of pneumothorax. The absence of pleural sliding in the second intercostal space on the LUS was defined as a small pneumothorax, whereas a large pneumothorax was recognized as the loss of sliding in both the second and third intercostal spaces (25).
Diaphragmatic excursion assessment
The anesthesiologists (Chen X and Na S) performed the diaphragmatic excursion assessment at the timepoints A and D due to the use of paralytics during the surgery. The low-frequency curvilinear transducer was placed in bilateral costal margins between the anterior and posterior axillary lines for longitudinal scanning. Images of ultrasonographic diaphragmatic excursions were obtained using the liver and spleen as acoustic windows during full respiration (Figure 1). With normal breathing of patients in the 45° semi supination position, the maximum vertical axis between the adjacent peaks and valleys using the sinusoid in the M-mode was measured as the diaphragmatic excursion movement (14). Offline analysis was also completed by Chen X and Na S.
Data collection
Each participant’s demographic data were collected. The data included sex, age, height, weight, BMI, American Society of Anesthesiologists (ASA) score, and lung function test data. Vital signs and hemodynamic index were recorded at all the study timepoints. At the timepoint B and C, mechanical ventilation parameters were noted, including Vt, respiratory rate, PEEP, FiO2, and peak inspiratory pressure. Outcomes included the duration of mechanical ventilation, OLV time, and the procedure length, total infusion details (the sum of the crystalline and colloidal liquids), length of stay (defined as the period from operation to discharge), and short-term complications according to postoperative routine CXR or CT findings before discharge.
Statistical analysis
The sample size was calculated using the G-Power software (version 3.1). Using previous publication for inference (4), a total of 80 patients were needed with the following assumptions: α error of 0.05, a β value equal to 0.2, a nonsphericity correction of 0.5, an effect size of 0.25, and a dropout rate set in 10% of patients. Continuous variables were described as the mean ± standard deviation or median and interquartile range after normality distribution testing and compared using a Student’s t-test or Mann-Whitney U-test as appropriate. Categorical variables were expressed as frequency and percentage, and comparison was made with Fisher’s exact test. The relationship between the changes in the diaphragmatic excursions and LUS scores was assessed by Spearman’s correlation. Repeated-measure one-way analysis of variance (ANOVA) was used to analyze the aeration changes in the ventilatory lungs. SPSS statistical software version 23.0 (IBM Corp, Armonk, NY, USA) was used for data analysis, and the level of statistical significance was set as a P value less than 0.05.
Results
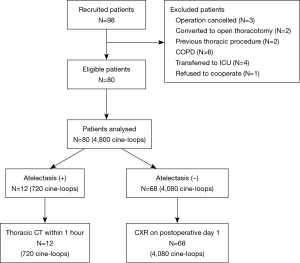
Patient participation and demographic information
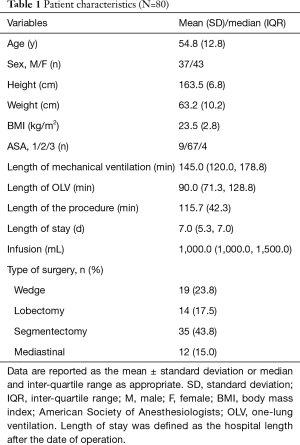
From January 2019 to May 2019, 98 adult patients were prospectively screened. After 18 patients met 1 or more exclusion criteria, a final total of 80 patients were enrolled (Figure 2). During the study, all of the ultrasound examinations of both the lungs and diaphragm were completed, and 4,800 cine-loops were stored. The demographic data of these patients are summarized in Table 1. Hemodynamic variables and ventilatory parameters are shown in Table 2.
Full table

Full table
LUS examination for pneumothorax and pleural effusions
Pneumothorax and pleural effusion were not imaged before anesthesia induction (timepoint A) and after intubation (timepoint B) in the LUS investigation because the surgery had not yet started. At the end of surgery (timepoint C), pneumothorax was detected in the operative sites of 76 patients (95.0%), with 23 cases being large pneumothorax and the others being small pneumothorax. Because the thorax was opened for VATS, the pneumothorax was deemed to be a residual gas. Due to chest tube drainage, the pneumothorax was imaged in 62 patients (78.8%) 15 minutes after extubation (timepoint D), with only 5 cases experiencing large pneumothorax. No pneumothorax was found on the non-operative side. Furthermore, small pleural effusions were discovered in 16 (20.0%) and 30 (37.5%) patients at the end of surgery and after extubation, respectively. All diagnosed effusions were in the dependent quadrants of the collapsed lung.
LUS examination for atelectasis
No atelectasis was discovered in the LUS examination before induction. Five minutes after induction, the LUS detected only irregular pleural lines and no typical atelectasis. At the end of the surgery, 12 patients had postoperative atelectasis in the posterior regions, yielding an incidence of 15.0% in our cohort; 4 cases were discovered in the lung resection group, and all were on the operative side; 8 cases were in the mediastinal tumor resection group and were distributed to their bilateral posterior quadrants. All 12 cases of postoperative atelectasis were confirmed by CT scan and remained in their dependent quadrants when scanned 15 minutes after extubation. No new atelectasis was discovered in the postoperative routine CXR in the LUS atelectasis-free group. There was no evidence of prolonged pneumothorax or pleural effusion, except for atelectasis, which had not completely disappeared on routine CXR at discharge. No complications such as bleeding, pneumonia, or infection were experienced by any participant.
Continuous LUS scores in non-surgical lungs
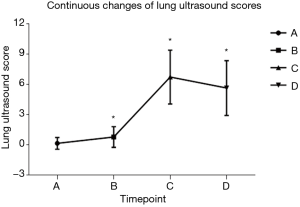
Serial scoring was only performed on non-surgical lung since LUS scores were inapplicable for pneumothorax. The mean LUS scores of the lungs undergoing ventilation before induction was 0.15±0.58. After the induction of general anesthesia, the score significantly increased to 0.78±1.02 (P<0.001). At the end of the surgery, the LUS score markedly jumped to 6.73±2.67 (P<0.001). Aeration improved after extubation when compared to the end of surgery (5.64±2.72, P<0.001) but was still higher than baseline (P<0.001) (Figure 4).
Preoperative and postoperative diaphragmatic excursion assessment on both sides
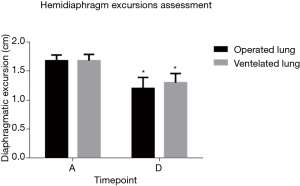
The diaphragmatic excursion on the operative side and contralateral sides were 1.68±0.10 and 1.68±0.11 cm before induction, respectively (P=0.562). After extubation, the excursion declined on both sides (1.20±0.19 cm, P<0.001; 1.30±0.16 cm, P<0.001) but was worse on the operative side (P<0.001) (Figure 5).
Relationship between LUS scores and diaphragmatic excursion assessment
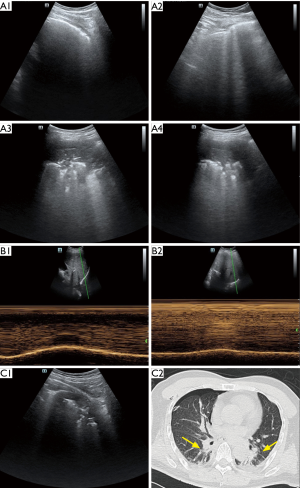
The changes in the LUS scores of the ventilated lungs were correlated with the changes in diaphragmatic excursion between the baseline and after extubation (r=−0.54; P<0.001). No correlation was found between the changes in LUS scores with age, BMI, duration of mechanical ventilation, or procedure. Perioperative temporal unilateral LUS signs of collapsed lung (Figure 6A), diaphragmatic excursion assessment of the homolateral side of the inferoposterior quadrant (Figure 6B), and signs of atelectasis in LUS and CT investigation (Figure 6C) of an atelectatic patient with mediastinal tumour resection are displayed in sequence in Figure 6.
Discussion
Our study showed that the LUS was feasible during all phases of the VATS perioperative period and was useful for tracking perioperative atelectasis and aeration loss. Diaphragmatic excursion assessment was also useful for diagnosing VATS aeration loss. Furthermore, it was confirmed that perioperative aeration loss was moderately correlated with diaphragmatic excursion changes. Few studies have quantified atelectasis after VATS in the immediate post-extubation or postoperative period, especially in the PACU. Therefore, to avoid patient transfers and exposure to cumulative radiation, we first proposed using a bedside ultrasound for the continuous monitoring of atelectasis and aeration loss at multiple timepoints in the perioperative period, even during ongoing VATS.
OLV is associated with nonspecific alveolar damage of the ventilatory lung due to high ventilation volume and high oxygen concentration toxicity (26). Meanwhile, a side-collapsed lung also suffers injuries from the interruption of lymphatic circulation during re-expansion, proinflammatory cytokine release, and ischemia-reperfusion injury (27). It is not always possible to open the collapsed lung completely, and postoperative atelectasis is often the result (28). The incidence rate of atelectasis (15.0%) on LUS was noted by the thoracic CT scan in our study and was higher than that in the previous report (29). These results were likely due to the earlier observational time (15 minutes after extubation), for which postoperative physiotherapy was not yet in place. Besides lung resection, our study also examined mediastinal tumor resection in the supine position. It was shown that the continuous changes in body position could help reduce atelectasis (30). Kang et al. showed that a continuous lateral rotation effectively reduced prevented ventilator-associated pneumonia and other pulmonary complications (31). These findings may explain the lowered frequency of atelectasis in the lung resection group compared to the mediastinal tumor resection group throughout which the supine position was used. The lateral decubitus position was shown to alleviate compression from the mediastinum and the heart and might also decrease alveolar closure and atelectasis (32).
The LUS score has been proven to significantly correlate with CT-measured lung reaeration in patients with atelectasis and pneumonia (10). As expected, the induction of general anesthesia caused a decrease in aeration, which was primarily located in the dependent areas (4). A pure oxygen content being used for mechanical ventilation intubation may account for this phenomenon, with either gas absorption or airway closure resulting from the preoxygenation (33). Sustained high FiO2, sigh breath disappearance, and prolonged dyskinesis are the main mechanisms for aeration loss during general anesthesia (34), with restoration of spontaneous breathing being shown to induce a marked improvement in aeration. Relief of the mediastinal compression and the influence of gravity allowed the ventilation and perfusion to slowly recover, but they did return to baseline values 15 minutes after extubation.
The present study demonstrates, for the first time, a correlation between changes in LUS scores and diaphragmatic excursions. Diaphragmatic excursions assessment via ultrasound has been widely used to identify severe respiratory dysfunction and to predict success in weaning patients from mechanical ventilation (35). Preoperative bilateral diaphragmatic excursions showed no differences, and the findings were consistent with normal diaphragmatic functions (36). In contrast to Subotic et al., our study showed a decrease in amplitudes of the contralateral diaphragm after lung resection (37). A possible explanation for this result is our earlier observation time (15 minutes after extubation) and our larger sample size (27 patients in the Subotic study). Patients showed decreased respiratory muscle endurance and inspiratory capacity from lost maximal muscle forces due to mechanical ventilation and surgery (38). These factors may explain the significant postoperative reduction in bilateral diaphragmatic excursions.
As a result of the limited diagnostic tools available to anesthesiologists in the intraoperative period, the role of LUS in the OR should be examined. During all phases of VATS, atelectasis can be first effectively detected at the end of surgery. As each patient is still on mechanical ventilation at that time, the performance of recruitment maneuver again may effectively reduce postoperative atelectasis. Both LUS score and diaphragmatic excursion could reflect ventilation; the variation of the two indexes would be helpful for patients adapting to and being weaned from mechanical ventilation. Our present study is only a tentative exploration, and understanding the significance of these two methods will be our aim for a future study.
Limitations
Our study has several limitations. First, the small number of mediastinal tumor resections in our cohort makes it difficult to draw conclusions. Future studies with a larger sample size are needed to explore the optimal position to reduce postoperative atelectasis in mediastinal tumor resection patients. Also, as pneumothorax was inapplicable of the LUS score, continuous aeration changes were only assessed in the non-operative lungs, and further studies on LUS scores in non-thoracic surgery are encouraged. Last, only patients with ultrasound-detected atelectasis received a CT scan. Whether patients without ultrasound diagnosis of atelectasis have a similar atelectasis sign in the CT scan of atelectasis is unknown.
Conclusions
In conclusion, our study demonstrated the feasibility of using LUS during all phases of the perioperative period in VATS. LUS offers the ability to diagnose and track perioperative atelectasis. Both LUS and diaphragmatic excursion assessment can facilitate continuous evaluation of aeration loss during VATS.
Acknowledgments
The authors thank Daqing Ma MD, PhD, Ying Chai MD, PhD, Lijian Huang MD, and Wenshan Li MD, for their valuable support and assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-19-595b
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-595b
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-595b). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the institutional review board of the Second Affiliated Hospital of Zhejiang University (I2018001364, 2018/12/05) and registered at ClinicalTrials.gov (no. NCT03802868) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhao Z, Wang W, Zhang Z, et al. Influence of tidal volume and positive end-expiratory pressure on ventilation distribution and oxygenation during one-lung ventilation. Physiol Meas 2018;39:034003. [Crossref] [PubMed]
- Borges JB, Amato MBP, Hedenstierna G. The increasing call for protective ventilation during anesthesia. JAMA surg 2017;152:893-4. [Crossref] [PubMed]
- Campos JH, Feider A. Hypoxia during one-lung ventilation-a review and update. J Cardiothorac Vasc Anesth 2018;32:2330-8. [Crossref] [PubMed]
- Monastesse A, Girard F, Massicotte N, et al. Lung Ultrasonography for the Assessment of Perioperative Atelectasis. Anesth Analg 2017;124:494-504. [Crossref] [PubMed]
- Sæverud HA, Falk RS, Dowrick A, et al. Measuring diaphragm movement and respiratory frequency using a novel ultrasound device in healthy volunteers. J Ultrasound 2019. [Crossref] [PubMed]
- Saccheri C, Morawiec E, Delemazure J, et al. ICU-acquired weakness, diaphragm dysfunction and long-term outcomes of critically ill patients. Ann Intensive Care 2020;10:1. [Crossref] [PubMed]
- Saleem Khan K, Meaney J, Martin-Loeches I, et al. MRI Assessment of Global and Regional Diaphragmatic Motion in Critically Ill Patients Following Prolonged Ventilator Weaning. Med Sci (Basel) 2019;7:66. [Crossref] [PubMed]
- Biasucci DG. Lung ultrasound in Intensive Care Unit: from knowledge gaps to future directions. Minerva Anestesiol 2017;83:672-4. [PubMed]
- Armbruster W, Eichholz R, Notheisen T. Lung Ultrasound for Anesthesia, Intensive Care and Emergency Medicine. AINS 2019;54:108-27. [PubMed]
- Yu X, Zhai Z, Zhao Y, et al. Performance of Lung Ultrasound in Detecting Peri-Operative Atelectasis after General Anesthesia. Ultrasound Med Biol 2016;42:2775-84. [Crossref] [PubMed]
- Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care 2017;21:325. [Crossref] [PubMed]
- Long L, Zhao H, Zhang Z, et al. Lung ultrasound for the diagnosis of pneumonia in adults: A meta-analysis. Medicine 2017;96:e5713. [Crossref] [PubMed]
- Galetin T, Defosse J, Schieren M, et al. Sensitivity of chest ultrasound for postoperative pneumothorax in comparison to chest X-ray after lung resecting surgery. Eur J Cardiothorac Surg 2020;57:846-53. [Crossref] [PubMed]
- Boon AJ, O’Gorman C. Ultrasound in the Assessment of Respiration. J Clin Neurophysiol 2016;33:112-9. [Crossref] [PubMed]
- Gil-Juanmiquel L, Gratacós M, Castilla-Fernández Y, et al. Bedside Ultrasound for the Diagnosis of Abnormal Diaphragmatic Motion in Children After Heart Surgery. Pediatr Crit Care Med 2017;18:159-64. [Crossref] [PubMed]
- Wei X, Li S, Cheng S, et al. Does daily chest ultrasound in the postoperative period contribute to an enhanced recovery after surgery pathway for patients undergoing general thoracic surgery? J Thorac Dis 2019;11:S1246-S1249. [Crossref] [PubMed]
- Mongodi S, Bouhemad B, Orlando A, et al. Modified Lung Ultrasound Score for Assessing and Monitoring Pulmonary Aeration. Ultraschall Med 2017;38:530-7. [Crossref] [PubMed]
- Markarian T, Zieleskiewicz L, Perrin G, et al. A lung ultrasound score for early triage of elderly patients with acute dyspnea. CJEM 2019;21:399-405. [Crossref] [PubMed]
- Lee JH, Choi S, Ji SH, et al. Effect of an ultrasound-guided lung recruitment manoeuvre on postoperative atelectasis in children: A randomised controlled trial. Eur J Anaesthesiol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Steinmetz P, Oleskevich S, Dyachenko A, et al. Accuracy of Medical Students in Detecting Pleural Effusion Using Lung Ultrasound as an Adjunct to the Physical Examination. J Ultrasound Med 2018;37:2545-52. [Crossref] [PubMed]
- Razazi K, Boissier F, Neuville M, et al. Pleural effusion during weaning from mechanical ventilation: a prospective observational multicenter study. Ann Intensive Care 2018;8:103. [Crossref] [PubMed]
- Maury É, Pichereau C, Bourcier S, et al. Diagnostic ultrasound in pneumothorax. Rev Mal Respir 2016;33:682-91. [Crossref] [PubMed]
- Santos-Silva J, Lichtenstein D, Tuinman PR, et al. The lung point, still a sign specific to pneumothorax. Intensive Care Med 2019;45:1327-8. [Crossref] [PubMed]
- Williamson JP, Grainge C, Parameswaran A, et al. Thoracic ultrasound: what non-radiologists need to know. Curr Pulmonol Rep 2017;6:39-47. [Crossref] [PubMed]
- Patella M, Saporito A, Puligheddu C, et al. Lung ultrasound to detect residual pneumothorax after chest drain removal in lung resections. Ann Thorac Surg 2018;105:1537-42. [Crossref] [PubMed]
- Bender SP, Anderson EP, Hieronimus RI, et al. One-Lung Ventilation and Acute Lung Injury. Int Anesthesiol Clin 2018;56:88-106. [Crossref] [PubMed]
- Lai G, Guo N, Jiang Y, et al. Duration of one-lung ventilation as a risk factor for postoperative pulmonary complications after McKeown esophagectomy. Tumori 2020;106:47-54. [Crossref] [PubMed]
- Charlesworth M, Glossop AJ. Strategies for the prevention of postoperative pulmonary complications. Anaesthesia 2018;73:923-7. [Crossref] [PubMed]
- Grossi W, Masullo G, Londero F, et al. Small incisions, major complications: video-assisted thoracoscopic surgery management of intraoperative complications. J Vis Surg 2018;4:12. [Crossref] [PubMed]
- Tusman G, Acosta CM, Bohm SH, et al. Postural lung recruitment assessed by lung ultrasound in mechanically ventilated children. Crit Ultrasound J 2017;9:22. [Crossref] [PubMed]
- Kang SY, DiStefano MJ, Yehia F, et al. Critical care beds with continuous lateral rotation therapy to prevent ventilator-associated pneumonia and hospital-acquired pressure injury: a cost-effectiveness analysis. J Patient Saf 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hewitt N, Bucknall T, Faraone NM. Lateral positioning for critically ill adult patients. Cochrane Database Syst Rev 2016;2016:CD007205.
- Östberg E, Auner U, Enlund M, et al. Minimizing atelectasis formation during general anaesthesia-oxygen washout is a non-essential supplement to PEEP. Ups J Med Sci 2017;122:92-8. [Crossref] [PubMed]
- Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care 2015;60:609-20. [Crossref] [PubMed]
- Tenza-Lozano E, Llamas-Alvarez A, Jaimez-Navarro E, et al. Lung and diaphragm ultrasound as predictors of success in weaning from mechanical ventilation. Crit Ultrasound J 2018;10:12. [Crossref] [PubMed]
- Kim K, Jang DM, Park JY, et al. Changes of diaphragmatic excursion and lung compliance during major laparoscopic pelvic surgery: A prospective observational study. PLoS One 2018;13:e0207841. [Crossref] [PubMed]
- Subotic DR, Stevic R, Gajic M, et al. Diaphragm motion and lung function prediction in patients operated for lung cancer – a pilot study on 27 patients. J Cardiothorac Surg 2013;8:213. [Crossref] [PubMed]
- Sferrazza Papa GF, Pellegrino GM, Di Marco F, et al. A Review of the Ultrasound Assessment of Diaphragmatic Function in Clinical Practice. Respiration 2016;91:403-11. [Crossref] [PubMed]


