Three cases of lung cancer in pregnancy and literature review
Introduction
The incidence of tumors during pregnancy is 1bincomprising 0.07–0.1% of all malignant tumors (1). Breast cancer, cervical cancer, melanoma, ovarian cancer, leukemia, and lymphoma, are the malignant tumors that occur most frequently during pregnancy. Lung cancer during pregnancy is rare, but it is the second leading cause of cancer-attributed mortality in women of childbearing age (2). In recent years, the increase in the number of women who smoke has seen a rise in the incidence of lung cancer. For pregnant women, smoking can carry increased risks, such as a delay in gestational age; furthermore, the incidence lung cancer during pregnancy has also gradually increased. This article reports three cases of lung cancer during pregnancy and reviews the relevant literature, to explore the diagnosis, treatment, and prognosis of pregnant women with lung cancer (Table 1).

Full table
Methods
We performed a retrospective review of records of pregnant patients with lung cancer seen at Beijing Friendship Hospital from 2013 to 2019. A total of three patients were included in the study. The clinical and pathological data of these patients were analyzed, including age, symptoms, signs, pathological types, clinical diagnosis, treatment process and prognosis. Also the relevant literature was reviewed. The study was approved by Ethics Committee at Beijing Friendship Hospital.
Case presentation
Case 1
The patient, 26 years old, was admitted to hospital in May 22, 2018. Regular obstetric examinations were carried out during the pregnancy.
At 30 weeks of gestation, the patient began to suffer hemoptysis with no obvious cause, occurring at 4–5 days intervals with about 1 mL expectorated per time. Slightly dark red blood clots with pink secretions were observed. The patient’s symptoms included occasional chest tightness and choking, low fever, night sweats, and wasting. At an extramural hospital visit, auscultation found the patient to have no abnormalities of heart or lungs. A chest examination revealed a high-density block shadow on the left lung. The patient was prescribed Zithromax to be taken orally for 1 week. But the hemoptysis became more frequent, occurring once every 1–2 days after 1 week of medication.
At a second extramural hospital visit on May 30th 2018 (4 days ago) the patient underwent a number of tests: sputum culture suggested Haemophilus parainfluenzae; sputum smear returned negative (weak acid-fast staining & fluorescence acid-fast staining); sputum smear for fungus showed no hypha or spores; sputum smear showed white blood cell (WBC) number <10/hp; and routine blood examination returned normal results. The patient was recommended to attend obstetric checks regularly and visit the pneumology clinic after childbirth. Due to the fetus being small, with little amniotic fluid (index 7.3 cm), the patient was admitted to our hospital for evaluation.
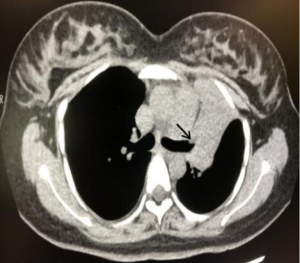
Examination showed a clear sound in both lungs when breathing and no rales. The fetus showed good movement and the ECG was normal. Dexamethasone was administered to promote fetal lung maturation. Blood gas, routine, and PCT were all normal. An anti-TB antibody test came back negative, as did Chlamydia pneumoniae IgM antibody and mycoplasma detection tests. The results of respiratory pathogen IgM tests were all negative. A sputum smear found gram-positive cocci but no acid-fast bacillus. Tumor marker tests indicated that AFP 394.02 ng/mL (0–15 ng/mL) was significantly elevated, while CEA, CA125, CA199, CA153 0–15 ng/mL, CYFRA211, CA724, NSE, and SCC were all normal. Further chest CT scans showed upper lobe of left lung atelectasis and bronchus truncation (Figure 1). In the mediastinum, the lymph nodes were enlarged, and the biggest one was about 1.1 cm diameter. After a full discussion within the department, pregnancy combined with lung cancer was considered to be highly likely.
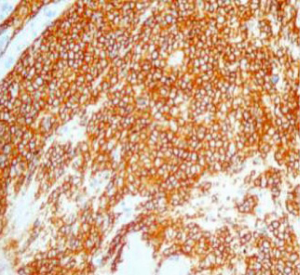
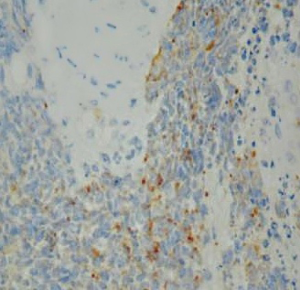
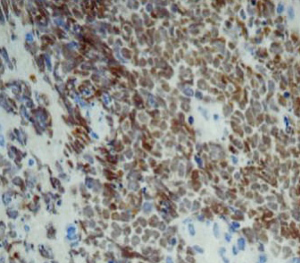
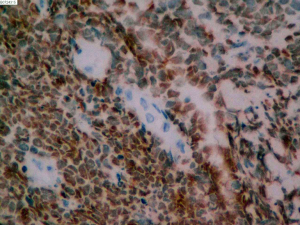
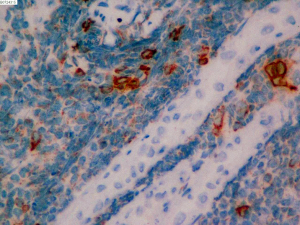
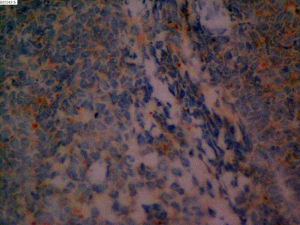
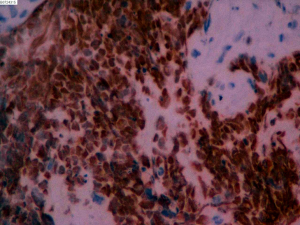
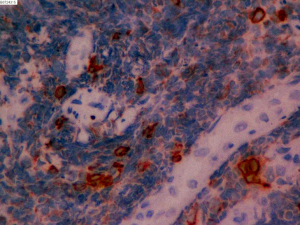
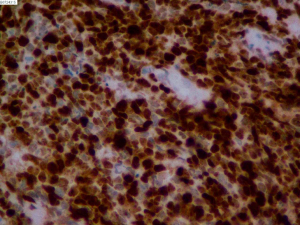
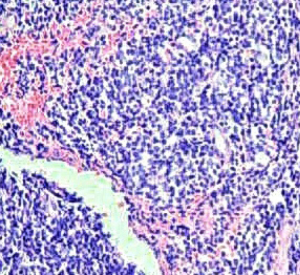
On June 5, 2018, 37 weeks into the pregnancy, the patient underwent low-segment cesarean section. The newborn weighed 2,450 g and was in a good condition. The patient made a good recovery and was transferred to the thoracic surgery ward on day 3 after surgery. Tracheoscopy examination revealed the upper lobe bronchus opening of left lung to be occluded, and new organisms were found. Pathological biopsy detected small round cells (Figure 2). Immunohistochemistry staining detected CD56+, CK+, Syn+, CgA+, Ki-67 approximately 90%+, CD3−, CD20−, LCA−, TDT−, and TTF-1+ (Figures 3-8). The diagnosis was lung small cell neuroendocrine carcinoma.
On June 12, 2018, further PET-CT examination found a soft tissue lump in the left hilum, partial density reduction, left lung upper lobe bronchus truncation, distal left upper lung consolidation, and significantly increased FDG metabolism. Malignant lesions (central lung cancer with possible necrosis) with distal obstructive atelectasis were considered. Lymphadenectasis in the mediastinum VI, left hilum, and between the left lobe suggested multiple lymph node metastasis. In summary, the diagnosis was small cell lung cancer in the left upper lobe (cT4N2M0), the clinical stage was phase III. It was recommended for the patient to undergo chemotherapy for 2–4 cycles, before receiving radiotherapy.
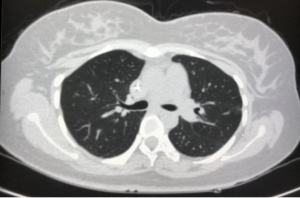
On June 19, 2018, the patient began intravenous chemotherapy of Etoposide 140 mg d1–d3+ Nedaplatin 30 mg d1–d3, after 2 courses of chemotherapy. The AFP levels in the patient’s blood dropped to 62.43 ng/mL, and chest CT suggested that the atelectasis, consolidation, and bronchial stenosis had improved (Figure 9). The lymph nodes in the left hilum and hilum decreased in size. No abnormalities were detected by cranial MRI examination. The patient received 6 complete courses of chemotherapy and underwent radiation therapy in December 2018. At 19 months after birth, the patient reported no discomfort, and regular imaging evaluations had revealed no lesions; her serum tumor markers were normal, and she had experienced no recurrence.
Case 2
A 39-year-old woman was admitted to hospital in July 7th 2014 for “20 weeks of menopause, cough and sputum with left back pain that had continued for 1 month and was aggravated for more than 10 days”. The patient had not received any physical examinations in the year before her pregnancy. The patient in this case did not undergo regular antepartum examinations. In 1993, the patient underwent extramural cesarean section due to cephalopelvic disproportion (CPD) and gave birth to a healthy baby. One month before admission, the patient developed a phlegmy cough for no apparent reason, with a blunt pain in the left chest and left back, which gradually became aggravated, especially when lying on her left side during the night. The patient occasionally experienced wheezing, but found that laying on her right side could alleviate her discomfort. At an extramural visit, a TB-Ab test returned negative results. Applied symptomatic treatment had no obvious effect; the amount of white mucus-like phlegm increased and the pain in the patient’s chest did not improve. Chest examination revealed pleural effusion on the left side. A pumping test highly suggested adenocarcinoma (ADCAC). The patient requested termination of the pregnancy. Chest CT revealed pleural effusion, swelling, and pneumonia on the left side, and lymphadenovarix in the mediastinum. During hospitalization, 800 mL of pleural effusion for examination was pumped, which indicated ADCAC. The patient was diagnosed with stage IV disease. On July 14th 2014, the baby was abandoned through lethal induction of labor. Intravenous chemotherapy (specifics unknown) was administered outside the hospital. Four months after induction, the patient died of tumor metastasis.
Case 3
The patient, aged 26, was admitted to hospital on May 13, 2013 for “22 weeks of menopause, with lung cancer found 4 days previously, and the patient requested induction of labor.” Applied regular examinations had taken place during the pregnancy. Two weeks before being admitted to hospital, the patient noticed masses on her neck and underwent CT examination in our hospital. CT scan revealed irregular soft tissue shadow on the right hilum and right upper lobe, and middle segment bronchial stenosis, along with multiple nodules in both lungs. Possible hilar lymph node, mediastinum lymph node and supraclavicular lymph node metastasis was suspected. Lymph node biopsy in the neck suggested adenoid epithelial nest infiltration, indicating adenocarcinoma lymph node metastasis. The results of immunochemistry suggested that the adenocarcinoma stemmed from partial cancer cells with neuroendocrine differentiation in the lungs. The immunohistochemistry results were as follows: CK (+), Ki-67 (30%+), CK8 (+), TTF-1 (+), TG (−), E-cad (+), ER (−), PR (−), c-erbb-2 (−), CK7 (+), CD56 (+/−), Syn (−), and CgA (−) (Figures 10-12). The patient’s clinical stage was stage IV. The patient did not experience chest tightness, cough, or hemoptysis. It was decided that the patient’s labor needed to be induced. Mifepristone was administered orally, ethacridine lactate was injected into the amniotic cavity to induce labor, and the process was smooth. After the induction, the patient applied extramural combined intravenous chemotherapy for five courses and received palliative radiation treatment for brain metastasis. On December 30th 2013, the patient died due to multiple metastasis and multiple organ failure.
Results
The three patients with lung cancer during pregnancy were all under 40 years old, and the gestational weeks of diagnosis were 20, 22 and 36 weeks, respectively. Two of the women were clinical stage IV and 1 was clinical stage III, all of which constitute advanced lung cancer. Pathological type differed between the women: 1 case was adenocarcinoma, 1 case was small cell neuroendocrine carcinoma, and the remaining case was adenocarcinoma with neuroendocrine carcinoma. Of the 3 patients, 1 had hemoptysis and 1 had cough and sputum with pain in the left chest and back, while 1 experienced no obvious symptoms. On examination, 1 patient had metastatic mass in the neck, and the remaining 2 patients had no obvious positive signs. Chest radiographs were performed in 2 cases, revealing a high-density massive mass in the left lung and a large amount of pleural effusion; all of the patients underwent CT scan of the lung, which detected atelectasis, bronchus truncation, pleural effusion, and irregular soft tissue next to the hilar. Two patients were diagnosed during the second trimester and had mid-term induction of labor. After lung cancer surgery in other hospitals, they were died at 4 and 7 months after diagnosis. The remaining patient, who was diagnosed at 36 gestational week underwent cesarean section at 37 weeks. The patient received chemoradiotherapy, and was still alive at 19 months postpartum follow-up.
Discussion
The first case of lung cancer in pregnancy was reported by Barr in 1953. Between 1957 and 2001, there were 14 cases of gestational lung cancer reported. To date, there have been about 66 reported cases (3-5), with an average age of 36 years old (range, 17–45 years old), an average gestational age of 27.3 weeks (range, 8–38 weeks), and a history of smoking in 60% of these cases (5). Almost all of these patients were diagnosed in the second trimester; 97% were at an advanced stage (stage III or IV), and more than 50% had distant metastasis (5). Non-small cell lung cancer accounted for 82% of the pathological types, among which adenocarcinoma and large cell undifferentiated carcinoma were the most common, and 18% of cases were small cell lung cancer (5). The overall prognosis was poor, with a mean survival of 7.5 months (range, 1–42 months) (6). The patients in our study were young: 2 cases were 26 years old, 1 case was 39 years old, and all were younger than 40 years old. The gestational weeks at diagnosis were 20, 22 and 36 weeks, respectively. It can be seen that the patients in our study were diagnosed before the second trimester of pregnancy, in contrast with other reported cases. Two cases were diagnosed at clinical stage IV, and 1 case at clinical stage III. Of the 3 patients in our study, 1 had lung adenocarcinoma, 1 had pulmonary small cell neuroendocrine carcinoma, and 1 had adenocarcinoma with neuroendocrine carcinoma.
The cause of lung cancer during pregnancy is unknown. Hatem found that 19 (61%) patients in their study of 31 gestational lung cancer cases had an active history of smoking (7). Among these lung cancer patients, 60% of them had continued to smoke after pregnancy. Therefore, smoking may be related to the occurrence of some tumors. On the other hand, Mitrou’s research showed that only 35% of pregnant lung cancer patients had a history of smoking, while 27% had no history of smoking at all (the other 38% had unknown smoking history) (5). Studies by Dagogo-Jack et al. also showed that 8 patients who were diagnosed with gestational lung cancer between 2009 and 2015 did not smoke or only smoked occasionally (8). Therefore, in addition to smoking, there may be other carcinogenic factors involved in the occurrence and development of lung cancer during pregnancy. All 3 of the patients in this study reported no history of smoking, and the pathogenesis is still unknown.
Nose et al. (9) found that in about 50% of lung adenocarcinoma tissue of the patients, expression levels of estrogen receptor of lung cancer, suggesting that it may be related to tumorigenesis. In vitro experiments conducted by Niikawa et al. (10) showed that estradiol can significantly increase the proliferation of ER-positive lung cancer cells, so it is speculated that the progression of lung cancer in pregnancy may be related to the increase in hormone levels. Hayama et al. (11) found the expression of ER significantly increase the prolto also be associated with poor prognosis. One of the 3 patients in this study underwent ER and PR tests, and the results were negative. The decreased expression level of PAPPA, and the activation of EGFR or ALK mutations may also be involved in the development and progression of lung cancer during pregnancy (5,12).
Symptoms and signs of lung cancer in patients can be related either to the primary tumor or distant metastasis. Common symptoms include a cough, hemoptysis, mode changes in coughing, wheezing, loss of appetite, and weight loss. Most patients already have distant metastasis at the time of diagnosis. The reasons for the delay in diagnosis may be attributed to the non-specific clinical manifestations of early-stage lung cancer. Clinicians often interpret fatigue, dyspnea, and coughing as being related to the pregnancy itself, rather than as tumor-related symptoms. Most consider a differential diagnosis of lung cancer until patients exhibit symptoms of advanced disease, such as hemoptysis, brain metastasis, or Horner’s syndrome. In addition, many patients worry about the effects of ionizing radiation and are reluctant to undergo imaging examination during pregnancy. This also causes a delay in diagnosis.
Some scholars have recommended that pregnant women over the age of 30 who are smokers should consider the possibility of lung cancer when respiratory symptoms occur during pregnancy (13). At this point, the patient’s medical history and symptoms should be inquired about in detail, and the patient should undergo thorough examination, to determine if there are enlarged lymph nodes, skin changes, abnormal breasts, or hepatosplenomegaly. A needle biopsy can be used to obtain a pathological diagnosis for superficial enlarged lymph nodes. The main symptom of 1 of patients in this study was hemoptysis, 1 patient experienced a cough with left front chest and back pain, and the other patient displayed no obvious symptoms. One patient was found to have a metastatic mass in the neck, and the remaining 2 patients had no significant positive findings. It is evident that the clinical symptoms and signs of lung cancer patients in pregnancy are not specific, which makes early clinical diagnosis difficult. For patients with recurrent respiratory symptoms, the necessary imaging studies should be performed promptly to confirm the diagnosis as soon as possible.
Radiation doses of less than 0.1 Gy (10 rads) for chest X-ray, CT, and MRI during pregnancy are safe with abdominal shielding protection (14). The chest lateral radiograph is often used for primary screening of lung cancer. Due to the potential teratogenicity of ionizing radiation, CT examinations should be avoided in the first trimester. MRI during pregnancy is relatively safe and is more sensitive than chest X-ray. PET examination can fully assess the extent of the disease, lymph nodes and distant metastasis, but due to the level of radioactivity, PET examination and bone scan during pregnancy should be avoided. Because sedation and anesthesia may possibly be required, bronchoscopy with biopsy is performed only occasionally during pregnancy. In some emergencies, it can be done in the third trimester, but careful fetal monitoring is vital (7). Two of the 3 patients in this study underwent chest radiography, suggesting a high-density shadow on the left lung and a large number of pleural effusions. Each of the 3 patients underwent pulmonary CT examination, which revealed atelectasis, bronchial truncation, pleural effusion and irregular soft tissue shadows around the hilum. One patient underwent PET-CT after delivery, which indicated central lung cancer with distal obstructive atelectasis and multiple lymph node metastasis. Microscopic biopsy pathology confirmed pulmonary small cell neuroendocrine cancer.
Treating lung cancer during pregnancy is challenging, and so far, there is no standard treatment. It has been reported that 51.4% of patients commence treatment after childbirth, with only 24% receiving treatment during pregnancy (5). Patients with stage I and stage II are eligible for thoracotomy, and stage III and stage IV are treated with chemotherapy. Radiotherapy may be considered after delivery.
With the advancements made in surgery, visual thoracoscopic surgery can also be carried out during pregnancy. During the operation, the mother and child should be monitored and postoperative analgesia should be applied actively. For early-stage lung cancer, surgical resection can be considered. Kim performed thoracoscopic resection on a pregnant patient with cancer in the right lower lobe at 24 weeks of gestation. No adverse reactions occurred in the mother or child during the operation. The patient gave birth to a healthy newborn after 37 weeks of pregnancy and was followed up for 19 months after surgery. The patient was alive and no recurrence or distant metastasis was found (15).
During the first trimester, the administration of chemotherapy is associated with high risk of miscarriage and malformation reaching 20%. This risk is reduced to 1% when chemotherapy is administered during the second and third trimesters, with a similar incidence to that of general population (7). The risks of chemotherapy for the fetus include growth restriction, premature birth, and anemia caused by myelosuppression. Most chemotherapeutic drugs have small molecular weight and can pass through the placenta. The chemotherapeutic drugs that cause fetal malformation mainly include antimetabolites and alkylating agents. Anthracyclines and alkaloids cannot easily penetrate the placenta due to their high molecular weight. At present, platinum-based combination chemotherapy is the first-line solution for lung cancer in pregnancy. Carboplatin is relatively safe in the gestation period due to its low toxicity. However, patients who are treated with cisplatin are more prone to fetal adverse events, such as intrauterine growth restriction, bilateral ventricular enlargement, respiratory distress syndrome, and fetal nephrotoxicity (16). Taxanes have also been shown to be safe when used during the second and third trimesters. Currently, no recommendations exist with regard to dosages of chemotherapy during pregnancy. Usually, the same dosages are used as for non-pregnant patients. Breastfeeding during chemotherapy is contraindicated because chemotherapeutic drugs are excreted with breast milk (17).
Jänne reported a case of woman who was 26 gestational weeks who received cisplatin plus vinorelbine combined chemotherapy for lung cancer. At 27 gestational weeks, the patient gave birth prematurely; no chemotherapy-related neonatal adverse complications were discovered (18). For non-small cell lung cancer, Saha et al. applied gemcitabine plus carboplatin chemotherapy, which resulted in only minor side effects and good tolerance (19). Azim applied carboplatin combined with paclitaxel for 5 consecutive weeks of peripheral therapy for advanced non-small cell lung cancer. The peak plasma concentration of paclitaxel was low; there was low toxicity in the mother (20). In Yates’ study, a patient who was diagnosed with lung cancer at 18 weeks of gestation received neoadjuvant chemotherapy (cisplatin + docetaxel) in the second trimester. Imaging showed complete remission. After 35 weeks of gestation, the patient received radical radiotherapy. After follow-up for 16 months, the patient is alive and no complications have been found in her child (21).
Delivery should take place after 32 weeks of gestation, when fetal lung development is more mature and at least 3 weeks after the last cycle of chemotherapy to ensure the resolution of maternal/fetal myelosuppression (22).
Radiotherapy is not administered during pregnancy, especially in the first trimester. In early pregnancy, radiotherapy is associated with fetal congenital malformations, nor is it recommended in the second trimester (14). Radiotherapy during pregnancy may lead to fetal developmental stagnation or organ malformation, and teratogenic doses are usually above 0.1–0.2 Gy (23). However, in special cases, such as cerebral metastasis or bone metastasis, palliative radiotherapy may be considered, and measures to protect the fetus should be taken (7).
In this study, 2 of the patients were diagnosed with lung cancer after the second trimester and were subsequently transferred to the hospital. The remaining patient was diagnosed at 36 weeks of gestation, and was administered Etoposide + Nedaplatin combined with chemotherapy after 37 weeks of cesarean section. To date, the patient has undergone 6 complete courses of chemotherapy; radiation therapy was performed in December 2018. At 19 months after the birth, the patient reported no discomfort, and regular reviews of imaging studies had revealed no lesions. The patient’s serum tumor markers were normal, and she had experienced no recurrence.
The overall prognosis of lung cancer during pregnancy is poor: 12% of patients die within 1 month after delivery, 70% of patients survive for only a few months, and only 19% survive for more than 12 months. Early-stage patients have a better prognosis (5,16). In this study, 2 patients died 4 months and 7 months after diagnosis, and both were clinical stage IV. The other patient was clinical stage III and was healthy 19 months after delivery.
In conclusion, although lung cancer is one of the most common malignant tumors, pregnancy with lung cancer is rare. Nevertheless, its incidence rate is increasing. Young women should be educated about the dangers of smoking and encouraged to quit before and during pregnancy. For patients with recurrent respiratory symptoms during pregnancy, imaging examination should be carried out promptly, and biopsy should be taken if necessary, to obtain early diagnosis. Pregnancy with lung cancer carries a high-risk. Platinum-based combination chemotherapy is safe in the second trimester of pregnancy. Regular ultrasound examination should be performed to monitor intrauterine growth and development, and there should be long-term follow-up after delivery.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-999
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-999). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee at Beijing Friendship Hospital (No.: 2020-P2-001-01). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma J, Yu L, Xu F, et al. Treatment and clinical outcomes of cervical cancer during pregnancy. Ann Transl Med 2019;7:241. [Crossref] [PubMed]
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23-47. [Crossref] [PubMed]
- Barr JS. Placenta metastases from a bronchial carcinoma. J Obstet Gynaecol Br Emp 1953;60:895-7. [Crossref] [PubMed]
- Bellido C, Barbero P, Forcén L, et al. Lung adenocarcinoma during pregnancy: clinical case and literature review. J Matern Fetal Neonatal Med 2019;32:3300-2. [PubMed]
- Mitrou S, Petrakis D, Fotopoulos G, et al. Lung cancer during pregnancy: A narrative review. J Adv Res 2016;7:571-4. [Crossref] [PubMed]
- Jackisch C, Louwen F, Schwenkhagen A, et al. Lung cancer during pregnancy involving products of conception and a review of the literature. Arch Gynecol Obstet 2003;268:69-77. [Crossref] [PubMed]
- Azim HA Jr, Peccatori FA, Pavlidis N. Lung cancer in the pregnant woman: To treat or not to treat, that is the question. Lung Cancer 2010;67:251-6. [Crossref] [PubMed]
- Dagogo-Jack I, Gainor JF, Porter RL, et al. Clinicopathologic features of NSCLC diagnosed during pregnancy or the peripartum period in the era of molecular genotyping. J Thorac Oncol 2016;11:1522-8. [Crossref] [PubMed]
- Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 2009;27:411-7. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Hayama M, Chida M, Tamura M, et al. Unexpected rapid growth of estrogen receptor positive lung cancer during pregnancy. Ann Thorac Cardiovasc Surg 2014;20:325-8. [Crossref] [PubMed]
- Pan H, Hanada S, Zhao J, et al. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS One 2012;7:e48799. [Crossref] [PubMed]
- Ceauşu M, Hostiuc S, Sajin M, et al. Gestational lung adenocarcinoma: case report. Int J Surg Pathol 2014;22:663-6. [Crossref] [PubMed]
- Sarıman N, Levent E, Yener NA, et al. Lung cancer and pregnancy. Lung Cancer 2013;79:321-3. [Crossref] [PubMed]
- Kim JW, Kim JS, Cho JY, et al. Successful video-assisted thoracoscopic lobectomy in a pregnant woman with lung cancer. Lung Cancer 2014;85:331-4. [Crossref] [PubMed]
- Boussios S, Han SN, Fruscio R, et al. Lung cancer in pregnancy: report of nine cases from an international collaborative study. Lung Cancer 2013;82:499-505. [Crossref] [PubMed]
- Rothschild SI. Lung Cancer in Pregnancy-A Forgotten Disease Entity. J Thorac Oncol 2016;11:1376-8. [Crossref] [PubMed]
- Jänne PA, Rodriguez-Thompson D, Metcalf DR, et al. Chemotherapy for a patient with advanced non-small cell lung cancer during pregnancy: a case report and a review of chemotherapy treatment during pregnancy. Oncology 2001;61:175-83. [Crossref] [PubMed]
- Saha A, Rudd R. Gemcitabine and carboplatin: is this the best combination for non-small cell lung cancer. Expert Rev Anticancer Ther 2006;6:165-73. [Crossref] [PubMed]
- Azim HA Jr, Scarfone G, Peccatori FA. Carboplatin and weekly paclitaxel for the treatment of advanced non-small cell lung cancer (NSCLC) during pregnancy. J Thorac Oncol 2009;4:559-60. [Crossref] [PubMed]
- Yates R, Zhang J. Lung Cancer in Pregnancy: An Unusual Case of Complete Response to Chemotherapy. Cureus 2015;7:e440. [PubMed]
- Pentheroudakis G, Orecchia R, Hoekstra HJ, et al. Cancer, fertility and pregnancy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v266-73. [Crossref] [PubMed]
- Orecchia R, Lucignani G, Tosi G. Prenatal irradiation and pregnancy: the effects of diagnostic imaging and radiation therapy. Recent Results Cancer Res 2008;178:3-20. [Crossref] [PubMed]