
Clinical characteristics and outcomes of patients with diffuse large B cell lymphoma treated with R-CHOP-like or CHOP-like regimens: an 8-year experience from a single center
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, characterized by aggressive and heterogeneous features. Although DLBCL can be classified into two categories, namely, germinal center B-cell-like (GCB) and non-germinal center B-cell-like (non-GCB), the standard therapy for these two subtypes is the chemotherapeutic regimen called CHOP, which comprises cyclophosphamide, doxorubicin, vincristine, and prednisone. In the last decade, the addition of rituximab (R), an anti-CD20 monoclonal antibody, to the standard chemotherapy regimen has dramatically improved the outcomes of patients with DLBCL (1). Since then, several studies have demonstrated the inability of the International Prognostic Index (IPI) to effectively predict the prognosis of patients with DLBCL. Hence, revised IPI (R-IPI) and National Comprehensive Cancer Network IPI (NCCN-IPI) were generated (2,3). However, the efficacy of these two indicators in prognosis prediction of patients with DLBCL was still insufficient. Although new indicators based on IPI have been proposed, their efficiency in prognosis prediction remains to be tested (4).
About 30–50% patients with DLBCL show resistance to, or relapse after, R-CHOP treatment (5,6). Chimeric antigen receptor (CAR) T cell therapy has been reported as a novel promising therapy for refractory or relapse (R/R) DLBCL patients. In clinical trials, the overall response rate of CAR-T cell therapy in R/R DLBCL patients was more than 80%, and a few patients achieved long-term remission (7,8). In general, the lack of standard techniques for T cell or peripheral blood mononuclear cell harvesting and CAR-T cell manufacturing and quality control may limit the clinical applications of this therapeutic regimen (9). The efficacy of novel target drugs in R/R DLBCL patients was not better than that of CAR-T cell therapy in clinical trials (10-17).
Here, we report the real-world data of the clinical characteristics and outcomes of patients with DLBCL that were treated with or without rituximab.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-19-589).
Methods
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the ethics committee of Fujian Medical University Union Hospital (No. 2019KJCX047). As this study performed retrospective data analysis and had no effect on patients’ treatments, patients’ consents were not obtained. Use of patients’ data and/or test results for this study was approved by the ethics committee of our institute.
Patients
Diagnosis was confirmed through tissue biopsy or surgical excision according to the World Health Organization (WHO) classification (18). Newly diagnosed patients with DLBCL who were 14 years or older and received no less than four cycles of immunochemotherapy or chemotherapy were included in the study. Patients with primary mediastinal lymphoma, primary central nervous system (CNS) lymphoma, or positive serology for human immunodeficiency virus were excluded. Data were collected from the medical records of patients included from 1 January 2011 to 31 December 2018 at Fujian Medical University Union Hospital, Fujian province, China.
Treatment and evaluation
Treatment response was evaluated according to the International Working Group Response Criteria for Malignant Lymphoma (19). Newly diagnosed patients with DLBCL were treated with CHOP-like or R-CHOP-like regimens. In case of disease progression or relapse, patients were treated with second-line regimens as recommended by NCCN guidelines, such as R-DHAP or R-DA-EPOCH (20).
Routine laboratory tests and clinical assessment were performed at the beginning of each treatment cycle. Interim assessment, including laboratory tests and imaging examinations, was performed no later than the sixth cycle. Treatment response was determined by interim assessment. Bone marrow biopsy was repeated at the interim assessment and at the end of treatment if initially involved.
Overall survival (OS) was defined as the time from the date of treatment inception to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time from the date of treatment inception to the date of disease progression, relapse, or death, whichever was reported first. Death from all causes was included. Survival time was measured until 10 June 2019.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 for Windows. Dichotomous and continuous variables were compared using the Chi-square test and t-test, respectively. Time-to-event data were analyzed using the Kaplan-Meier method, and differences between them were analyzed using the log-rank test. Variables known to be significant prognostic factors in the univariate analysis were further used for multivariate analysis. Two-sided P values <0.05 were considered statistically significant.
Results
Demographic and clinical characteristics of patients
Between January 2011 and December 2018, 570 patients with newly diagnosed DLBCL who met the inclusion criteria were included in this study. The demographic and clinical characteristics of these patients are listed in Table 1. Their median age at diagnosis was 55 [14–86] years. Of them, 351 (61.6%) were male, 145 (25.4%) had B symptoms, 261 (45.8%) had serum lactate dehydrogenase (LDH) levels higher than normal, 125 (21.9%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 2–4, and 182 (31.9%) had more than one extranodal disease site. As the imaging data for two patients before treatment were insufficient for precise staging, only 568 patients could be staged. Among them, 359 (63.0%) were in advanced stages (III–IV). IPI scores for 568 patients were as follows: 231 (40.7%) low-risk, 122 (21.5%) low-intermediate-risk, 132 (23.2%) high-intermediate-risk, and 83 (14.6%) high-risk. As classified by NCCN-IPI, 107 (18.8%) patients were low-risk, 257 (45.2%) were low-intermediate-risk, 158 (27.8%) were high-intermediate-risk, and 46 (8.1%) were high-risk. Detection of serum macroglobulin (β2-MG) level was performed in 353 patients, of which 88 (24.9%) had serum β2-MG levels higher than normal.
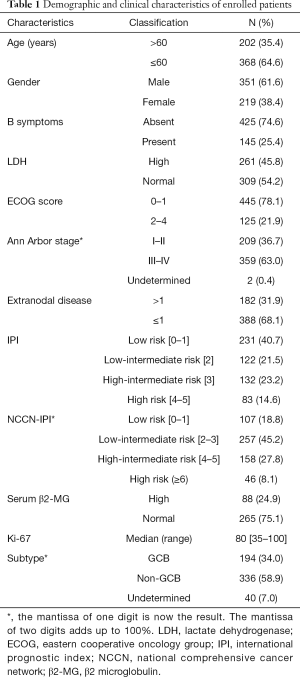
Full table
The expression of the proliferative marker, Ki-67, was detected in the tissues of 481 patients. The median positive rate of Ki-67 in tumor cells was 80%, and the positive rate of Ki-67 in tumor cells was higher than 50% in 461 of 481 samples. According to the Hans Criteria, 194 (34.0%) patients were classified into GCB subtype and 336 (58.9%) into non-GCB subtype; 40 (7.0%) patients could not be classified, as their biopsy tissues were insufficient to perform immunohistochemical detection (Table 1).
Treatment and response of patients
All 570 patients enrolled received no less than four cycles of CHOP-like or R-CHOP-like regimens. Of them, 133 patients who received less than three cycles of rituximab were assigned to the CHOP-like regimen. The remaining 437 patients were assigned to the R-CHOP-like regimen. Patients were assessed for response after 2–5 cycles of initial therapy using the Revised Response Criteria for Malignant Lymphoma (8).
The overall response rate was 83.3%, and 67 (11.8%) patients achieved stable disease (SD) or suffered from progressive disease (PD) after receiving CHOP-like or R-CHOP-like regimen. Response to chemotherapy or immunochemotherapy could not be assessed in 28 patients, as their imaging assessments were incomplete. A total of 156 (27.3%) patients became primarily refractory to the therapeutic regimen, suffered from PD, or relapsed after achieving complete response (CR) during treatment or follow-up. Further, 34 patients received different types of cell therapy as follows: 24 received autologous stem cell transplantation (SCT), 2 received allogeneic SCT, and 8 received CAR-T cell therapy. Radiation was administered to 45 patients because they had residual disease after chemotherapy or immunochemotherapy or suffered from PD or relapse with CNS involvement (Table 2).
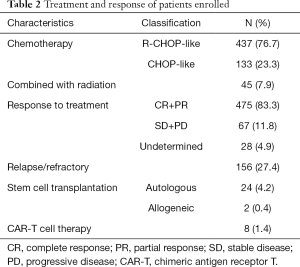
Full table
Survival and prognostic factors
The median follow-up period for the entire group was 25.77 months (range, 2.73–102.33 months), and 100 patients died. The median OS and PFS were not reached in the entire group (Figure 1).
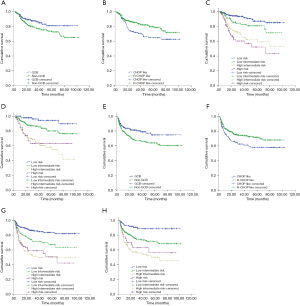
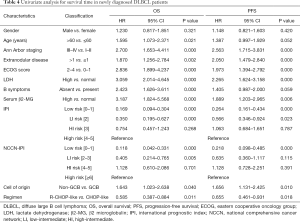
Kaplan-Meier curve and univariate analysis showed that GCB subtype, R-CHOP-like treatment, Ann Arbor stage I-II, not more than 1 extranodal disease site, ECOG score ≤1, normal serum LDH level, normal serum β2-MG level, absence of B symptoms, and lower IPI or NCCN-IPI score were associated with longer OS and PFS, and were favorable prognostic factors for OS and PFS. Age ≤60 years was associated with longer OS and served as a favorable prognostic factor for OS but not PFS (Tables 3,4, Figure 1). Kaplan-Meier curves for OS and PFS showed convergence of high-intermediate-risk and high-risk subgroups classified by both IPI and NCCN-IPI (Figure 1).
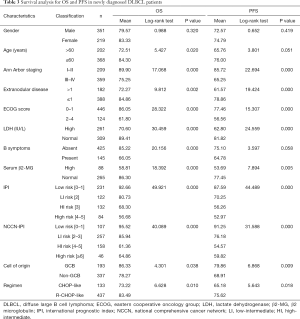
Full table
Full table
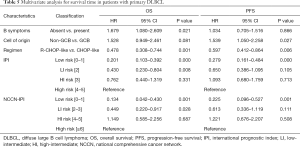
In the multivariate analysis, absence of B symptoms and R-CHOP-like treatment were independent favorable prognostic factors for OS. GCB subtype and R-CHOP-like treatment similarly served as independent favorable factors associated with PFS (Table 5). IPI and NCCN-IPI scores had no prognostic values for OS between high-intermediate- and high-risk subgroups. Moreover, IPI and NCCN-IPI scores had no prognostic values for PFS among low-intermediate-, high-intermediate-, and high-risk subgroups.
Full table
Discussion
DLBCL accounts for 31% and 40–50% of newly diagnosed NHL in developed countries and China, respectively. According to its origin, DLBCL may be classified into two subtypes GCB and non-GCB by immunochemistry or three subtypes by gene expression profiling, including GCB, activated B-cell (ABC), and unclassified (UNC) (21). Several studies have shown that the survival of patients with non-GCB subtype was worse than that of patients with GCB subtype (22,23). A similar phenomenon has been reported in patients with ABC subtype (24). Other characteristics that were either included or excluded in IPI were associated with the prognosis of patients with DLBCL (4,25-27). In the last decade, the use of rituximab was shown to improve the treatment response and survival of patients with DLBCL (1,28,29). However, long-term follow-up data for newly diagnosed patients with DLBCL in the real-world setting are scarce. Therefore, in the present study, we investigated the clinical characteristics and outcomes of newly diagnosed patients with DLBCL treated at our single center.
We found that most patients were not more than 60 years, male, and had non-GCB subtype. Most patients had IPI scores 2 and NCCN-IPI scores ≤3. ECOG score for most patients was less than 2. Serum β2-MG levels were higher than normal in some patients, while the positive rate of Ki-67 expression in most patients was higher than 80%. These results are similar to those reported by other studies from China (30,31). Survival analysis showed that some clinical characteristics such as age >60 years, Ann Arbor stage III–IV, extranodular disease >1 site, ECOG score 2–4, serum LDH >245 IU/L, serum β2-MG >3 g/L, presence of B symptoms, and non-GCB subtype were associated with shorter PFS and/or OS, and were unfavorable prognostic factors for patients with DLBCL in our study. Similar results have been observed by other researchers worldwide (28,30-32).
IPI score served as the most important prognostic indicator for non-Hodgkin lymphoma, including DLBCL. It comprises age, Ann Arbor staging, extranodular disease, ECOG score, and serum LDH level. Several reports have demonstrated that risk stratification based on IPI in DLBCL is of limited value, owing to the inclusion of rituximab to standard chemotherapy (2). Therefore, NCCN-IPI was proposed and applied to many studies in recent years (3). We analyzed the role of these two indices in determining the prognosis of patients with DLBCL who received regimens containing rituximab. According to both IPI and NCCN-IPI, patients could be divided into four groups as follows: low-risk, low-intermediate-risk, high-intermediate-risk, and high-risk. Although IPI and NCCN-IPI were independent prognostic factors for all patients with DLBCL in our study, univariate and multivariate analyses showed us that IPI and NCCN-IPI had limited prognostic values for the OS and/or PFS of patients that are at higher risk, especially high-intermediate- and high-risk patients. These results declined the prognostic values of IPI and NCCN-IPI for patients with DLBCL who received no less than three cycles of rituximab. A new index GELTAMO-IPI combining serum β2-MG levels with NCCN-IPI has been recently proposed (4). As we lacked data on serum β2-MG in approximately 40% patients, we could not objectively evaluate this index or compare it with other indices.
The R-CHOP-like regimen is the first-line regimen for DLBCL. However, some patients still could not receive sufficient doses of rituximab. In the early period of our study, most patients received CHOP-like regimens and could not use rituximab owing to financial constraints. However, in the later stage, most patients received CHOP-like regimens due to hepatitis B virus (HBV) infection. Patients treated with R-CHOP-like regimens had longer PFS and OS than others. Furthermore, multivariate analysis showed that the use of regimens comprising rituximab was an independent favorable prognostic factor for patients with DLBCL, consistent with the previously reported result (1,29-31). This observation provides further evidence of the use of rituximab for patients with DLBCL. Antibody-dependent cell-mediated cytotoxicity (ADCC) targeting CD20 induced by rituximab may enhance the antitumor effect of CHOP-like regimens owing to the expression of CD20 antigen on the membrane of B lymphocytes. Thus, prophylactic antiviral therapy was deemed effective along with rituximab in DLBCL patients with HBV infection (33,34). Recent studies have shown that different expression levels of antiapoptotic BCL-2 family proteins in DLBCL cells, existence of cancer stem-like cells, and high expression of CD47 antigen contribute to the chemosensitivity of CHOP-like or R-CHOP-like regimens. Therefore, detection of the expression of certain genes in DLBCL tissues may be useful for the selection of R-CHOP-like or CHOP-like regimen and addition of small-molecule inhibitors in patients with DLBCL (35-37). In our study, radiation was an essential supplement for patients who had CNS or testis involvement in the course of treatment or residual disease after completion of treatment.
Several reports have demonstrated primary refractory, secondary refractory, and relapse in 30–50% of DLBCL patients after comprehensive treatment. Survival of these patients was worse than that of other patients without these complications (5,6,38). The ratio of R/R in patients with DLBCL was not more than 30% in our study. Most patients were at low- and low-intermediate-risk, thereby contributing to this phenomenon. Hematopoietic SCT was considered as an effective approach for DLBCL patients with high-risk, poor prognosis, and relapse/refractory disease (39). However, some patients could not benefit from this method in our study. Therefore, the mechanisms underlying R/R DLBCL and development of therapeutic methods remain to be elucidated.
Several studies have shown that small-molecule inhibitors such as selective inhibitors of nuclear export, dual mTORC1/2 inhibitors, and aurora A kinase inhibitors alone or in combination with chemotherapy exhibited antitumor effects in patients with R/R DLBCL. However, these inhibitors were not more effective than CAR-T cell therapy, a novel cellular immunotherapy (10-17,40-42). No patient in our study participated in clinical trials of small-molecule inhibitors. As far as CAR-T cells are concerned, T cells are genetically modified to express an artificial receptor comprising an antigen recognition domain, a co-stimulatory domain, and an intracellular signaling domain to facilitate recognition of a specific antigen expressed on tumor cells (43). Two products of CAR-T cells, axicabtagene ciloleucel (axi-cel) and tisagenlecleucel, targeting CD19 specifically expressed on B lymphocytes have been shown to be effective for R/R B cell malignancies with high response rates and potential cure (44,45). However, limitations related to T cell harvesting and CAR-T cell manufacturing, disease progression during manufacturing, and resistance to CAR-T cell therapy have impeded their clinical applications (9,46). Only eight patients received CAR-T cell therapy and all of them achieved PR and survived; however, the long-term effects remain to be studied, as some patients with R/R DLBCL relapsed after receiving CAR-T cell therapy (44,45).
In conclusion, rituximab has been proven to be an important drug to improve the survival of patients with DLBCL. The prognostic values of IPI and NCCN-IPI have declined for patients with DLBCL in the rituximab era. New prognostic indices, thus, need to be proposed and validated to accurately and precisely predict the prognosis of patients. Novel prognostic markers based on molecular or cytogenetic aberrance may be more effective than others in prognostic prediction. New therapeutic approaches such as CAR-T cell therapy and novel drugs have demonstrated significant efficacy in anti-lymphoma treatment of relapse/refractory DLBCL patients and could serve as powerful strategies for treating DLBCL patients in future.
Acknowledgments
Funding: This work was supported by Fujian Provincial Health Technology Project (grant number: 2019-CX-15 and 2017-CX-16), the Joint Funds for the Innovation of Science and Technology, Fujian province (grant number: 2018Y9028), the University-Industry Cooperation Project of Fujian Science and Technology Department, Fujian, China (grant number: 2018Y4004), Construction project of Fujian Medical Center of Hematology (Min201704) and National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, China (22010301).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-19-589
Data sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-589
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-589). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Fujian Medical University Union Hospital (No. 2019KJCX047). As this study performed retrospective data analysis and had no effect on patients’ treatments, patients’ consents were not obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salles G, Barrett M, Foa R, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther 2017;34:2232-73. [Crossref] [PubMed]
- Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007;109:1857-61. [Crossref] [PubMed]
- Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42. [Crossref] [PubMed]
- Montalbán C, Diaz-Lopez A, Dlouhy I, et al. Validation of the NCCN-IPI for diffuse large B-cell lymphoma (DLBCL): the addition of beta2 -microglobulin yields a more accurate GELTAMO-IPI. Br J Haematol 2017;176:918-28. [Crossref] [PubMed]
- Ayers EC, Li S, Medeiros LJ, et al. Outcomes in patients with aggressive B-cell non-Hodgkin lymphoma after intensive frontline treatment failure. Cancer 2020;126:293-303. [Crossref] [PubMed]
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800-8. [Crossref] [PubMed]
- Li C, Zhang Y, Zhang C, et al. Comparation of CART19 and autologous stem-cell transplantation for refractory/relapsed non-Hodgkin's lymphoma. JCI Insight 2019;5:e130195. [Crossref] [PubMed]
- Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018;132:777-81. [Crossref] [PubMed]
- Graham C, Jozwik A, Pepper A, et al. Allogeneic CAR-T Cells: More than Ease of Access? Cells 2018.7. [PubMed]
- Ma X, Li L, Zhang L, et al. Apatinib in Patients with Relapsed or Refractory Diffuse Large B Cell Lymphoma: A Phase II, Open-Label, Single-Arm, Prospective Study. Drug Des Devel Ther 2020;14:275-84. [Crossref] [PubMed]
- Coffey GP, Feng J, Betz A, et al. Cerdulatinib Pharmacodynamics and Relationships to Tumor Response Following Oral Dosing in Patients with Relapsed/Refractory B-cell Malignancies. Clin Cancer Res 2019;25:1174-84. [Crossref] [PubMed]
- Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol 2019;37:481-9. [Crossref] [PubMed]
- Forero-Torres A, Ramchandren R, Yacoub A, et al. Parsaclisib, a potent and highly selective PI3Kdelta inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood 2019;133:1742-52. [Crossref] [PubMed]
- Kelly KR, Friedberg JW, Park SI, et al. Phase I Study of the Investigational Aurora A Kinase Inhibitor Alisertib plus Rituximab or Rituximab/Vincristine in Relapsed/Refractory Aggressive B-cell Lymphoma. Clin Cancer Res 2018;24:6150-9. [Crossref] [PubMed]
- Eyre TA, Hildyard C, Hamblin A, et al. A phase II study to assess the safety and efficacy of the dual mTORC1/2 inhibitor vistusertib in relapsed, refractory DLBCL. Hematol Oncol 2019;37:352-9. [Crossref] [PubMed]
- Ben-Barouch S, Kuruvilla J. Selinexor (KTP-330) - a selective inhibitor of nuclear export (SINE): anti-tumor activity in diffuse large B-cell lymphoma (DLBCL). Expert Opin Investig Drugs 2020;29:15-21. [Crossref] [PubMed]
- de Vos S, Swinnen LJ, Wang D, et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol 2018;29:1932-8. [Crossref] [PubMed]
- Swerdlow SH CE, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [Crossref] [PubMed]
- Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw 2019;17:650-61. [Crossref] [PubMed]
- Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology 2018;50:74-87. [Crossref] [PubMed]
- Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol 2008;26:4587-94. [Crossref] [PubMed]
- Robinson SD, Gabriel J, Webb A, et al. Immunohistochemical prognostication in diffuse large B-cell lymphoma: a single center 6-year retrospective analysis. Leuk Lymphoma 2015;56:2975-7. [Crossref] [PubMed]
- Read JA, Koff JL, Nastoupil LJ, et al. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: a meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clin Lymphoma Myeloma Leuk 2014;14:460-7.e2. [Crossref] [PubMed]
- Deng Y, Chen X, Huang C, et al. EZH2/Bcl-2 Coexpression Predicts Worse Survival in Diffuse Large B-cell Lymphomas and Demonstrates Poor Efficacy to Rituximab in Localized Lesions. J Cancer 2019;10:2006-17. [Crossref] [PubMed]
- Larousserie F, Kebe D, Huynh T, et al. Evidence for IL-35 Expression in Diffuse Large B-Cell Lymphoma and Impact on the Patient's Prognosis. Front Oncol 2019;9:563. [Crossref] [PubMed]
- Li X, Sun X, Li J, et al. Interim PET/CT based on visual and semiquantitative analysis predicts survival in patients with diffuse large B-cell lymphoma. Cancer Med 2019;8:5012-22. [Crossref] [PubMed]
- Wu JQ, Song YP, Su LP, et al. Three-year Follow-up on the Safety and Effectiveness of Rituximab Plus Chemotherapy as First-Line Treatment of Diffuse Large B-Cell Lymphoma and Follicular Lymphoma in Real-World Clinical Settings in China: A Prospective, Multicenter, Noninterventional Study. Chin Med J (Engl) 2018;131:1767-75. [Crossref] [PubMed]
- Wu J, Song Y, Su L, et al. Rituximab plus chemotherapy as first-line treatment in Chinese patients with diffuse large B-cell lymphoma in routine practice: a prospective, multicentre, non-interventional study. BMC Cancer 2016;16:537. [Crossref] [PubMed]
- Liu P, Han Y, Jiang SY, et al. A retrospective analysis of real-world outcomes of elderly Chinese patients with diffuse large B-cell lymphoma. Chin Med J (Engl) 2019;132:1807-14. [Crossref] [PubMed]
- Shi Y, Han Y, Yang J, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res 2019;31:152-61. [Crossref] [PubMed]
- Yang H, Wu M, Shen Y, et al. Treatment Strategies and Prognostic Factors of Primary Gastric Diffuse Large B Cell Lymphoma: A Retrospective Multicenter Study of 272 Cases from the China Lymphoma Patient Registry. Int J Med Sci 2019;16:1023-31. [Crossref] [PubMed]
- Chen KL, Chen J, Rao HL, et al. Hepatitis B virus reactivation and hepatitis in diffuse large B-cell lymphoma patients with resolved hepatitis B receiving rituximab-containing chemotherapy: risk factors and survival. Chin J Cancer 2015;34:225-34. [Crossref] [PubMed]
- Liu WP, Wang XP, Zheng W, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma 2016;57:1355-62. [Crossref] [PubMed]
- Chen J, Ge X, Zhang W, et al. PI3K/AKT inhibition reverses R-CHOP resistance by destabilizing SOX2 in diffuse large B cell lymphoma. Theranostics 2020;10:3151-63. [Crossref] [PubMed]
- de Jong MRW, Langendonk M, Reitsma B, et al. Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma. Int J Mol Sci 2019;20:6036. [Crossref] [PubMed]
- Bouwstra R, He Y, de Boer J, et al. CD47 Expression Defines Efficacy of Rituximab with CHOP in Non-Germinal Center B-cell (Non-GCB) Diffuse Large B-cell Lymphoma Patients (DLBCL), but Not in GCB DLBCL. Cancer Immunol Res 2019;7:1663-71. [Crossref] [PubMed]
- Vannata B, Conconi A, Winkler J, et al. Late relapse in patients with diffuse large B-cell lymphoma: impact of rituximab on their incidence and outcome. Br J Haematol 2019;187:478-87. [Crossref] [PubMed]
- Epperla N, Hamadani M, Reljic T, et al. Upfront autologous hematopoietic stem cell transplantation consolidation for patients with aggressive B-cell lymphomas in first remission in the rituximab era: A systematic review and meta-analysis. Cancer 2019;125:4417-25. [Crossref] [PubMed]
- Hong JY, Yoon DH, Suh C, et al. Bendamustine plus rituximab for relapsed or refractory diffuse large B cell lymphoma: a multicenter retrospective analysis. Ann Hematol 2018;97:1437-43. [Crossref] [PubMed]
- Lenz G, Hawkes E, Verhoef G, et al. Single-agent activity of phosphatidylinositol 3-kinase inhibition with copanlisib in patients with molecularly defined relapsed or refractory diffuse large B-cell lymphoma. Leukemia 2020. [Crossref] [PubMed]
- Rhodes J, Landsburg DJ. Small-Molecule Inhibitors for the Treatment of Diffuse Large B Cell Lymphoma. Curr Hematol Malig Rep 2018;13:356-68. [Crossref] [PubMed]
- Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17:147-67. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31-42. [Crossref] [PubMed]
- Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 2019;16:372-85. [PubMed]


