Anlotinib or platinum-pemetrexed as second-line therapy in EGFR T790M-negative lung cancer
Introduction
For advanced non-small cell lung cancer (NSCLC) patients harboring the activating mutant epidermal growth factor receptor (EGFR), EGFR tyrosine kinase inhibitors (TKIs) are the standard first-line therapy (1,2). Nevertheless, essentially all the patients who respond to the treatment will develop acquired resistance. The diversity of EGFR-TKI failure could be categorized into three modes (3). EGFR-TKI continuation is preferred in a subsequent setting for gradual progression. For local progression, continuation of EGFR-TKI as a systemic treatment plus local intervention is rational in clinical practice. For patients with aggressive progression after EGFR-TKI failure, chemotherapy or osimertinib are recommended depending on the acquired mutation. At the time of aggressive progression, 60% or above of patients are found to have the T790M mutation in their EGFR (4,5). The presence of T790M mutation reduces the binding of first-generation or second-generation EGFR-TKIs to the ATP-binding pocket of EGFR, thereby reducing EGFR-TKI–mediated inhibition of downstream signaling and potentially leading to disease progression. The standard therapy for patients who acquired the T790M mutation after first-line TKIs is osimertinib which has been confirmed in ARUA3 (6). How to manage T790M-negative patients after first-line EGFR-TKI failure continues to be controversial. In most cases, platinum-based chemotherapy is highly recommended (7) and continuation of TKI combined with chemotherapy has not been shown to benefit the patients (8).
Anlotinib is a novel multi-target TKI for tumor angiogenesis and tumor cell proliferation. The major targets include vascular endothelial growth factor receptor type 2 and 3 (VEGF 2, 3), platelet-derived growth factor-β (PDGFR-β), fibroblast growth factor receptor (FGFR), and stem cell-factor receptor (c-Kit). Anlotinib has been approved by the National Medical Products Administration (NMPA) based on the ALTER 0303 trial in China (9) as a third-line or beyond treatment for advanced NSCLC.
Anlotinib has showed manageable toxicity and potent antitumor potential in the treatment of advanced NSCLC with widespread clinical use. With the advancement of novel treatments, such as targeted therapy, oral medications have become more popular instead of chemotherapy for tumor control. For T790M-negative patients who are resistant to chemotherapy after first-line EGFR-TKIs treatment fails, anlotinib is an option. The impact of anlotinib as a second-line therapy compared with platinum-based chemotherapy in T790M-negative patients after first-line EGFR-TKIs failure remains unclear. Our retrospective study was to investigate the efficacy of anlotinib and platinum-pemetrexed chemotherapy inT790M-negative NSCLC patients after first-line EGFR-TKIs therapy.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-105).
Methods
Patients
We collected the clinical data of stage IV NSCLC patients who had failed first-line EGFR-TKIs therapy at Shanghai Chest Hospital from July 2018 to July 2019. The study protocol was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1935) and was in accordance with the Helsinki Declaration (as revised in 2013). Because the study is a retrospective study, and no harm is done to the patient, the requirement for informed consent was waived. The major inclusion criteria included (I) patients who were diagnosed with stage IV NSCLC histopathologically with the EGFR mutation (exon 19 deletion or L858R); (II) disease progression after first-line EGFR-TKIs therapy; (III) EGFR T790M-negativewas confirmed using the cobas EGFR Mutation Test(Roche Molecular Systems) after first-line EGFR-TKIs treatment failed; (IV) patients whose radiologic and laboratory data (before and during anlotinib/chemotherapy therapy) were available; (V) patients who received anlotinib or platinum-pemetrexed as the second-line treatment after first-line EGFR-TKIs therapy. The major exclusion criteria included (I) EGFR T790M-positive or unknown after first-line EGFR-TKIs therapy; (II) patients who had severe and/or uncontrolled diseases before anlotinib/chemotherapy therapy may result in discontinuing of second-line therapy.
Clinical assessments
In this retrospective study, we enrolled 20 patients who were given anlotinib after first-line therapy failed and another 42 patients who received platinum-pemetrexed chemotherapy (about 1:2 ratio) as a control group. All the patients were confirmed T790M-negative on the cobas EGFR Mutation Test, while disease progression after first-line EGFR-TKIs therapy. Patients were given anlotinib (12 mg) once daily from day 1 to day 14 (21 days as a course) until disease progression or intolerance was observed. In the chemotherapy cohort, intravenous pemetrexed (500 mg per square meter of body-surface area) plus either carboplatin [target area under the curve 5 (AUC5)] or cisplatin (75 mg per square meter) every 3 to 4 weeks for four to six cycles was administered. Patients without disease progression after four to six cycles of platinum therapy plus pemetrexed continued at pemetrexed maintenance dose until disease progression or intolerance. Baseline and follow-up chest CT examinations were performed for every patient before and during therapy. Follow-up CT examinations were performed every two courses to evaluate the response to anlotinib or chemotherapy. The Response Evaluation Criteria in Solid Tumors (RECIST v1.1) were applied to assess responses to therapy. The follow-up ended on December 9th, 2019.
Statistical analysis
Objective response rate (ORR) was defined as the portion of patients who achieved complete response (CR) and partial response (PR). Progression-free survival (PFS) was calculated from the beginning of the second line therapy to disease progression or the last follow-up visit. SPSS22.0 statistical software (IBM, Armonk, NY, USA) was used for statistical analyses. The logistic regression model was used to detect the clinical characteristics related to PFS. Kaplan-Meier method and the log-rank test were used to analyze PFS. P values <0.05 were considered statistically significant.
Results
Patient characteristics
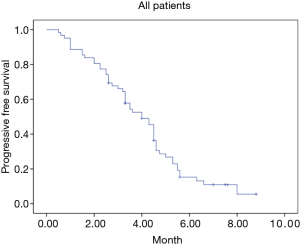
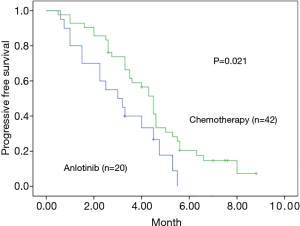
A total of 62 stage IV NSCLC patients without EGFR T790M mutation confirmed using the cobas EGFR Mutation Test (Roche Molecular Systems) after first-line EGFR-TKIs therapy were enrolled in our retrospective study from July 2018 to July 2019. All the patients were diagnosed with adenocarcinoma before the first-line EGFR-TKIs treatment. Demographic and clinical characteristics of all patients are shown in Table 1. At the endpoint, the median follow-up for all patients was 7.3 months. The median PFS of the second-line therapy among all patients was 4.0 months (Figure 1). Progression events occurred in 17 patients (85%) in the anlotinib group and 35 (83%) in the platinum-pemetrexed group. The duration of PFS was significantly longer in the platinum-pemetrexed group than that in the anlotinib group (median, 4.5 vs. 3.0 months; HR, 1.972; 95% CI, 1.078 to 3.607; P=0.021) (Figure 2).
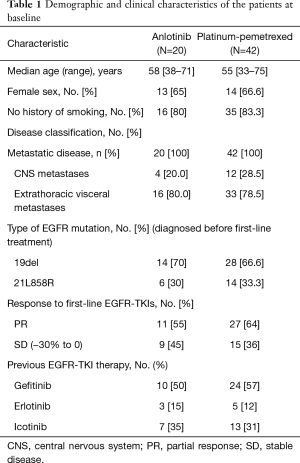
Full table
ORR and disease control rate
The response to anlotinib therapy included PD (30%), SD (55%), and PR (15%). The response rate was significantly better in the platinum-pemetrexed group (30.9%) than that in the anlotinib group (15%), and the disease control rate (DCR) of both group was 70% and 83%, respectively. Among the patients who responded to, disease progression or death was found in 17 of 20 patients (85%) in the anlotinib group and in 35 of 42 patients (83.3%) in the platinum-pemetrexed group. The overall survival analyses were not completed at the end point. Responses to treatment are shown in Table 2.
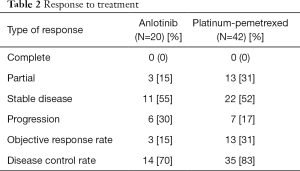
Full table
Safety and adverse events
The most common AEs with an incidence of ≥5% in the anlotinib group were: hypothyroid (60%), hypertension (55%), hand-foot syndrome (50%), fatigue (45%), decreased appetite (35%), oral mucositis (25%), proteinuria (20%), diarrhea (15%), and hoarseness (13.6%). Only hypothyroid, hypertension and deceased appetite were assessed as grade 3–4 with an incidence of 5% (Table 3).
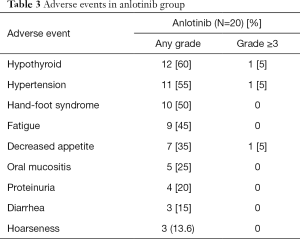
Full table
Discussion
In this retrospective study, we found that stage IV NSCLC patients without the T790M mutation who received anlotinib had worse response rates and a shorter PFS than those who received platinum therapy plus pemetrexed after first-line EGFR-TKIs therapy.
To our best knowledge, in patients with T790M-positive NSCLC after disease progression with first-line EGFR-TKIs therapy, osimertinib was more effective than combination platinum-based chemotherapy (6). For those patients without the T790M mutation, there are some options for disease management. Afatinib-cetuximab has shown approximately a 30% response rate (10). The immune checkpoint inhibitor (ICI)-directed therapy is available but its efficacy in EGFR-mutated lung cancer may be less than that observed in cancers with high mutation load such as tumors associated with heavy smoking history. The effect of PD1/PDL1 inhibitors in EGFR-mutated lung remains controversial. ICIs were less effective for NSCLCs with EGFR mutations than for lung cancers with the wild-type EGFR, and some may suffer from hyperprogressive disease (11). As evidence emerges on immunotherapy, further studies are needed to determine if the combination of ICI and anti-angiogenesis is useful for EGFR T790M-negative NSCLC patients .Currently, platinum-based chemotherapy itself remains a reasonable alternative. The current National Comprehensive Cancer Network guide-lines recommend platinum-based combination therapy for progression after failure of EGFR-TKIs first-line without the T790M mutation. HANSHIN Oncology Group 0109suggested this regimen as a possible platinum-based combination after EGFR-TKI failure (7).
Anlotinib is a novel multi-TKI similar to sorafenib and sunitinib, all of which target tumor angiogenesis and tumor cell growth, invasion, and metastasis. Anlotinib as a third-line or beyond treatment for advanced NSCLC has been approved by NMPA based on the ALTER 0303 trial (9). Anlotinib is also approved in China for the treatment of patients with soft-tissue sarcoma or as a third-line or beyond treatment for extensive-stage small cell lung cancer. The effect of anlotinib as a second-line therapy compared with platinum-based chemotherapy in T790M-negative patients after first-line EGFR-TKIs failure remains unclear. Our retrospective study did not find anlotinib to be superior to the platinum-based chemotherapy. The response rate was significantly better in the platinum-pemetrexed group (30.9%) than that in the anlotinib group (15%). The duration of PFS was significantly longer in the platinum-pemetrexed group than that in the anlotinib group (median, 4.5 vs. 3.0 months). As a third-line or beyond therapy, anlotinib has not shown advantages in terms of chemotherapy compared to being placed in the second-line. In T790M-negative patients after TKIs failed, bypass resistance mechanisms include using alternative cellular pathways and activating downstream signal transduction, which facilitate tumor cell growth and survival. MET, PI3KCA, HER2 gene mutations are most frequently observed (12). So the non-selective use of multi-target TKI, e.g., anlotinib as a second-line treatment may lead to failure.
The toxicity profile of anlotinib was similar to other analogous such as sorafenib, sunitinib, and regorafenib (13-15). In the anlotinib group, no hemoptysis was reported. The most frequent adverse events observed were hypothyroid (60%), hypertension (55%), hand-foot syndrome (50%), fatigue (45%), decreased appetite (35%) and oral mucositis (25%). One patient suffered from hypothyroid of grade 3 which was well controlled by taking levothyroxine sodium. Hypertension, which was well controlled by anti-hypertensive drugs, was the other adverse event with an incidence of 5% of grade 3. Severely decreased appetite was reported in one patient which led to dose reduction to 10 mg daily. Compared to the AEs associated with anlotinib reported in previous studies, no new AEs were observed in the present study (9,16). However, our results show that anlotinib was well tolerated in patients with late-stage NSCLC. All the adverse events appeared to be manageable. The safety profile in the platinum-pemetrexed group was consistent with that observed in the similar cisplatin-pemetrexed study like IMPRESS and AURA 3 trial (6,8). Overall, adverse events tended to be more severe in the platinum-pemetrexed group due to the longer treatment duration.
Our study has several limitations. First, the OS data required longer follow-up. Second, it was a retrospective study and the sample size was small. Third, due to the high false negative rates of the plasma ctDNA T790M test, a biopsy is recommended for patients with plasma T790M-negative results as well as disease progression after receiving first-line EGFR-TKIs. However, the feasibility of detecting the EGFR T790M mutation from plasma ctDNA samples, in line with previous reports (6,17,18).
Unfortunately, we find that as a second-line therapy, anlotinib was inferior to platinum-pemetrexed to patients with T790M-negative after disease progression with first-line EGFR-TKIs therapy. In recent studies, multi-TKI for tumor angiogenesis and tumor cell proliferation with the combination of ICI, could optimize the immunosuppressive tumor-associated macrophages and potentially increase the therapeutic response to immunotherapy both in lung cancer in vivo models and from preliminary clinical data in NSCLC (19). The proper combination may show promising anti-cancer activity than single agent. Abnormal angiogenesis and immunosuppression in the tumor microenvironment are barriers to effective cancer immunotherapy. Antiangiogenesis can have immune modulatory effects. The efficacy of ICI may be enhanced through the addition of antiangiogenesis to reverse VEGF-mediated immunosuppression. The combination of antiangiogenesis and immunotherapies in multiple tumor types have observed promising antitumor effects, inducing greater tumor vascular normalization and converted tumor microenvironment from immunosuppressive to immunosupportive (20). So in the further study, we will try to combine anlotinib plus ICI in T790M-negative patients to determine the antitumor effects.
In conclusion, anlotinib was less effective than platinum-pemetrexed chemotherapy in T790M-negative NSCLC patients with disease progression after failing the first-line EGFR-TKIs therapy.
Acknowledgments
We would like to thank all of investigators for their involvement in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-105
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-105
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-105
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-105). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was explicitly approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1935). Because the study is a retrospective study, and no harm is done to the patient, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan DSW, Yom SS, Tsao MS, et al. The international association for the study of lung cancer consensus statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: status in 2016. J Thorac Oncol 2016;11:946-63. [Crossref] [PubMed]
- Morita S, Okamoto I, Kobayashi K, et al. Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2009;15:4493-8. [Crossref] [PubMed]
- Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Hattori Y, Satouchi M, Shimada T, et al. A phase 2 study of bevacizumab in combination with carboplatin and paclitaxel in patients with non-squamous non-small-cell lung cancer harboring mutations of epidermal growth factor receptor (EGFR) after failing first-line EGFR-tyrosine kinase inhibitors (HANSHIN Oncology Group 0109). Lung Cancer 2015;87:136-40. [Crossref] [PubMed]
- Mok TSK, Kim SW, Wu YL, et al. Gefitinib plus chemotherapy versus chemotherapy in epidermal growth factor receptor mutation-positive non-small-cell lung cancer resistant to first-line gefitinib (IMPRESS): overall survival and biomarker analyses. J Clin Oncol 2017;35:4027-34. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Janjigian YY, Smit EF, Groen HJM, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer 2019;137:113-22. [Crossref] [PubMed]
- Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol 2011;22:1812-23. [Crossref] [PubMed]
- Porta C, Gore ME, Rini BI, et al. Long-term safety of sunitinib in metastatic renal cell carcinoma. Eur Urol 2016;69:345-51. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Jenkins S, Yang JCH, Ramalingam SS, et al. Plasma ctDNA Analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol 2017;12:1061-70. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res 2019;7:630-43. [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]